Abstract
Aims
To investigate the site of barrier function to the passive diffusion of a small molecule (phalloidin) in the corneal epithelium in the mouse.
Methods
Penetration of phalloidin (molecular weight 1115 daltons) into the cornea was evaluated by studying fluorescent binding of phalloidin to actin in tissue sections, in whole mount preparations, and in the fixed intact globe by confocal microscopy. In addition, the location of tight junction proteins in the individual layers of the corneal epithelium was determined by immunohistochemistry.
Results
Phalloidin staining of corneal sections was positive in all corneal layers in tissue sections and in all layers of the corneal epithelium except the suprabasal layer in excised fixed whole mounts of the cornea. However, when phalloidin staining was attempted in intact fixed globes, before excision of the cornea for whole mount preparation, only the most superficial layer of cells was stained indicating that phalloidin could not penetrate the tissue beyond the suprabasal epithelial layer. Detergent (Triton X‐100) treatment of the excised cornea and the intact fixed globe, allowed penetration of phalloidin into the suprabasal epithelial layer. Tight junction proteins occludin, ZO‐1 and claudin were present in most layers of the cornea but while ZO‐1 and occludin were distributed in a typical pericellular pattern, claudin seemed to be particularly prominent in the suprabasal layer and appeared only as a discontinuous punctate pericellular pattern in the superficial layer. Intraepithelial leukocytes were detected in the superficial epithelium and the basal epithelium but not in the suprabasal epithelium.
Conclusion
The suprabasal epithelium cell layer appears to represent the main barrier site to the passage of small molecules and cells in the mouse cornea and this property may be attribuatable to prominent claudin expression in this layer.
The corneal epithelium presents a barrier to the external world both for fluid and particulate material including cells. In this sense it forms part of the immunological barrier to invasion provided by the skin. Barrier function in most epithelial and endothelial layers is provided by intercellular tight junctions.1 These junctions form the regulated semipermeable barrier in the paracellular space.2 The corneal epithelium presents a unique barrier since it is bathed in tear fluid, which provides it with at least part of its nutrition. In addition, the basal layer of the epithelium is constantly regenerating and therefore as cells divide there may be the possibility that junctions loosen and the barrier function is temporarily disrupted. The tight junction complex in corneal epithelium includes the integral transmembrane proteins occludin, variable numbers of claudins and junction adhesion molecule‐1 (JAM‐1). In addition, there are membrane‐associated (peripheral) proteins such as ZO‐1, ZO‐2, and ZO‐3 present in the tight junction plaque, many of which contain the PDZ domain (Psd/SAP90, discs large, Z0‐1).1,2,3 The peripheral proteins are capable of interacting directly with the cytoplasmic domain of occluding.4
Previous studies showed an association of F‐actin with tight junction.5,6,7 It is widely asumed that the actin filaments found in the tight junction participate in regulation of tight junction permeabilisation7,8,9,10 and that actin can bind to occludin through the ZO proteins.11 Tight junctions also determine apical‐basal polarity in epithelial cells. Initiation of tight junction assembly requires calcium ions and E‐cadherin‐mediated cell‐cell contact, while later stages of tight junction assembly are regulated by a series of proteins containing PDZ domains.1 These include the PAR‐3/aPKC/PAR‐6 complex which mediates the binding of PAR‐3 to JAM‐1 while aPKC phosphorylates ZO‐1, occludin‐1 and claudin‐1. Other proteins such as cingulin, α and β catenin, and vinculin mediate binding of tight junction complexes to F‐actin filaments via JAM‐1, ZO‐1, ZO‐2 and ZO‐3. It has been also suggested, that actin filaments function as a contracting “purse string” during epithelial wound healing.12
We were interested to investigate the cellular relationships of the corneal epithelium not only of the intraepithelial layers but also its relationship with stromal cells particularly cells with an invasive potential. This is of special relevance in view of recent findings indicating that there is a rich population of CD45 leukocytes in the corneal stroma13,14 and corneal epithelium.15 In addition, the tear fluid is constantly enriched with migratory leukocytes derived from leaky vessels in the conjunctiva and lacrimal apparatus.16
In this study we examined mouse corneal epithelium prepared as whole mounts. Previous studies have suggested that tight junctions occur only in the superficial layer of epithelium since ZO‐1 and ‐2 antibody staining occurred in this layer. Interestingly, staining for claudin‐1 was located in the basal and suprabasal cells.17 However, our results using confocal microscopy of mouse corneal whole mounts and intact eyes, suggest that the epithelial permeability barrier is more likely located in the suprabasal layer and not in the basal or superficial layer of the corneal epithelium. This barrier prevents small molecules from traversing in either direction between the corneal stroma and the tear fluid. In addition, since corneal leukocytes were observed in the basal and superficial layers but not the suprabasal layer, we suggest that the suprabasal layer is the major barrier site preventing trafficking of cells across the different corneal layers under physiological conditions.
Materials and methods
Animals
Inbred female C57BL/6 (H‐2b), 8–10 weeks old were obtained from the Medical Research Facility at the Medical School of Aberdeen University. All animals were managed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and under the regulations of the United Kingdom Animals (Scientific Procedures) Act 1986 (UK).
Preparation of tissue
Wholemount corneas
Mice were euthanised and corneas were immediately fixed in situ with 4% paraformaldehyde in phosphate‐buffered saline (PBS) for 20–30 min. The entire cornea was then excised at the limbus under the operating microscope and further fixed for 1 h in 4% paraformaldehyde. The tissues were then washed in PBS and either directly stained with antibodies (see below) or were permeabilised with 0.3% Triton X‐100 for 1 h at room temperature and then stained.
Whole eye study
Whole eyes were removed and fixed with 4% paraformaldehyde diluted in PBS for 1 h. Eyes were then washed in PBS and directly stained or first permeabilised with 0.3% Triton X‐100 for 1 h.
Corneal sections
Whole eyes were removed, embedded in optimum cutting temperature (O.C.T.) medium and frozen. 8 μm thick sections were then cut in a cryostat.
For immunohistology the sections were fixed with acetone for 20 min and allowed to dry overnight.
Phalloidin localisation
Tissues were prepared as above in order to maximise detection of phalloidin. Each of the fixed tissues was incubated with solutions containing phalloidin to best achieve evidence of its access to the different layers of the cornea by passive diffusion, as follows (a) excised corneas (b) intact whole eyes (c) intact whole eyes after Triton X‐100 treatment. Each tissue was then processed and stained as below.
Antibodies
The staining procedures were performed with the following antibodies: purified rat anti mouse ZO‐1, rabbit anti‐claudin and rabbit anti‐occludin (Zymed Laboratories, South San Francisco, CA, USA, dilution 1:50), rat anti‐mouse CD45 (30‐F11) (BD Pharmingen, San Diego, USA, dilution 1:100), biotinylated rabbit anti‐rat immunoglobulins (DakoCytomation, Glostrup, Denmark, dilution 1:100), biotinylated porcine anti‐rabbit immunoglobulin (DakoCytomation, 1:100). Streptavidin conjugated with rhodamine (TRTC) and with FITC were purchased from Jackson Immunoresearch Laboratories, West Grove, PA, USA, (dilution 1:50). For F‐actin staining BODIPY 558/568 phalloidin was used (Molecular Probes, Inc., dilution 1:20).
Immunohistology
Wholemount corneas were prepared as described above. To block nonspecific staining, corneas were first incubated with strain specific serum for 20 min at 37°C. They were then incubated overnight at 4°C in 100 μl of diluted primary antibody or isotype‐matched control immunoglobulin diluted in PBS. The tissue was then washed three times for 10 min each in PBS and incubated with secondary biotinylated antibody for 2 h at room temperature, washed three times (10 min each) in PBS and finally incubated with fluorescently labelled streptavidin and washed again. Four radial cuts were performed using a sharp razor blade and corneas were then mounted in mounting medium (VECTASHIELD or VECTASHIELD–propidium iodide) with the epithelial side upward in 18×18 mm wells made of nail polish on glass slides and covered with a coverslip. For staining for F‐actin, corneas were washed immediately after fixation (or permeabilisation) in PBS and incubated with BODIPY 558/568‐phalloidin for 2 h at room temperature, washed 3 times (10 min each) in PBS and then either mounted or stained as described above.
The staining procedure of the whole eye was the same as in wholemount corneas, only after the last wash the corneas were excised at the limbus under the microscope and mounted.
Frozen sections of the eyes were prepared, nonspecific staining was blocked with strain specific serum and eye sections were then stained with primary antibody or with phalloidin diluted in PBS for 1 h, and then washed three times 5 min each in PBS. Sections were then incubated with secondary biotinylated antibody for 1 h, washed again 3×5 min and then incubated with fluorescently labelled Streptavidin for 30 min. This was followed by a final wash (3×5 min) and the slides were mounted in mounting medium—VECTASHIELD, or VECTASHIELD‐PI—and covered with a coverslip. All steps were performed at room temperature.
At least four different corneas were examined in each experiment. All experiments were repeated at least twice. For negative controls we used isotype‐matched immunoglobulins.
Confocal microscopy
Wholemount corneas and frozen eye sections were analysed using a confocal Laser Scanning Microscope (LSM Meta, Zeiss, Gottingen Germany). An oil‐immersion objective (40×) was used to obtain individual images. To obtain orthogonal section displays of whole corneal thickness or of corneal epithelium, series of multiple Z‐sections were generated. Images were created using the LSM software. To count the number of positively labeled cells in the corneal epithelium, a series of multiple Z‐sections were generated and single images created for each sample. Positively labeled cells were then manually counted in the merged images. Different regions of the wholemount corneas were analysed—namely the central, paracentral and the peripheral region and expressed as cells per mm2 (mean ± SD).
Results
F‐actin staining of mouse cornea
Corneal sections
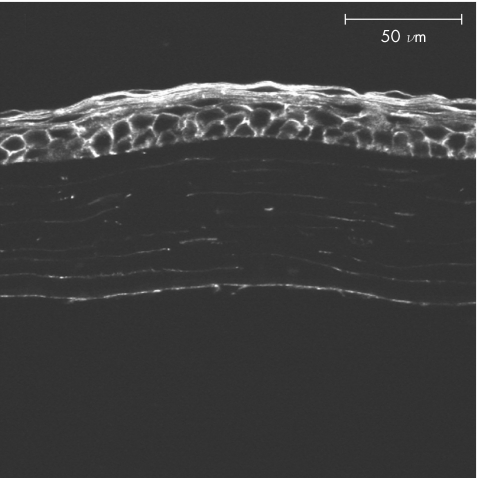
In order to show the distribution of F‐actin in the normal mouse cornea, we first examined frozen sections. Frozen sections showed uniform intensity of F‐actin staining in all layers of the corneal epithelium (fig 1).
Figure 1 Phalloidin stained section of normal mouse cornea showing a uniform distribution of F‐actin in all layers of epithelium, in the stroma and endothelium.
Wholemount preparations of mouse cornea
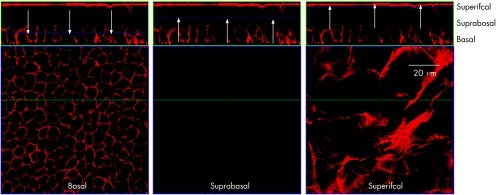
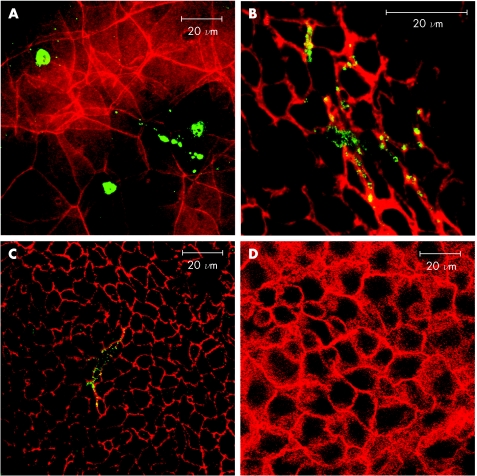
We next examined excised mouse corneal wholemounts stained for F‐actin. Orthogonal images revealed that all layers of the mouse cornea (endothelium, stroma, basal layer and superficial layer of the epithelium) were strongly positive for F‐actin except the suprabasal layer of epithelium. No positive staining was observed between the basal layer and the superficial layer of the mouse cornea creating an apparent discontinuity in the epithelium (fig 2).
Figure 2 Orthogonal reconstruction of corneal epithelium from non‐permeabilised corneal wholemount stained with phalloidin. The top section of each panel presents a confocal “optical section” (Z axis), while the lower level represents the “en face” view. The arrows indicate the depth of section corresponding to the en face view in each panel. The basal and superficial layer of the epithelium showed strong positive staining for F‐actin. No staining was detected in the suprabasal layer creating an apparent discontinuity in the epithelium.
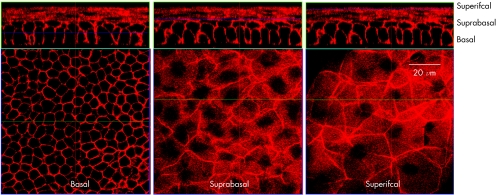
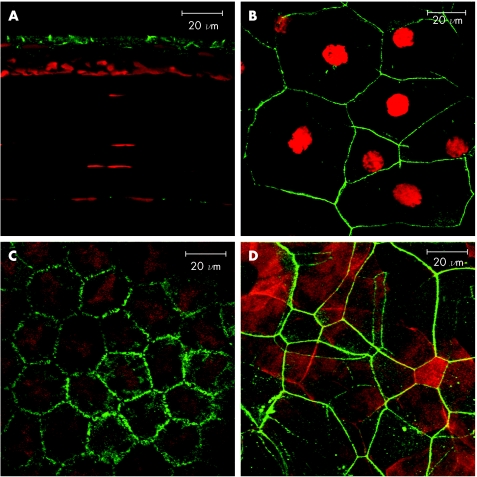
We next attempted to demonstrate F‐actin in whole mounts by permeabilisation of the epithelial layers with and without 0.3% Triton X‐100 for one hour prior to staining. Using this method, F‐actin positivity of the suprabasal cell layer was evident. Moreover, the remaining layers of the cornea were appropriately stained in permeabilised corneal whole mounts (fig 3).
Figure 3 Orthogonal reconstruction showing more detailed views of corneal epithelium from Triton X‐100 permeabilised corneal wholemount stained with phalloidin. All three layers of the epithelium—basal, suprabasal and superficial—stained strongly for F‐actin as shown in the respective lower panels by confocal optical sections of the individual layers.
Whole eye study
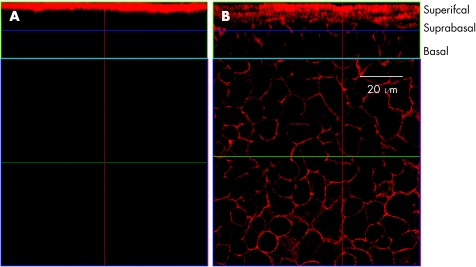
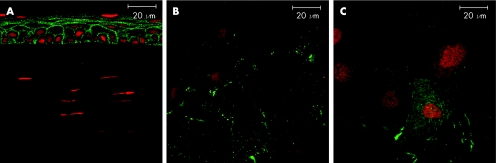
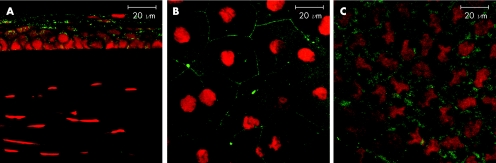
In order to determine whether the lack of suprabasal layer staining for F‐actin in corneal whole mounts as opposed to sections, was due to failure of phalloidin to penetrate and stain all the corneal epithelial layers by passive (lateral) diffusion, as is the case with fixed cut sections of the tissue (figs 1 and 2), we incubated whole, intact eyes in phalloidin‐containing medium. We compared both Triton X‐100 permeabilised and non permeabilised intact, whole fixed eyes. Corneas from the unpermeabilised eyes showed F‐actin staining only in superficial layers of epithelium with no staining in any other epithelial layer (fig 4A). More importantly, no positive staining was found in the stroma or the endothelium. In contrast, Triton‐X permeabilised eyes stained all layers of epithelium (fig 4B), and also demonstrated some actin staining of corneal stromal and endothelial cells although less than in excised whole mount preparations or in sections.
Figure 4 Whole eye study Wholemount preparation of both permeabilised and non‐permeablilised intact whole eyes stained for F‐actin. (A) non‐permeabilised eyes showed F‐actin staining only in superficial layers of epithelium with no staining in any other corneal layer (B) corneas from the permeabilised eyes revealed uniform staining of the basal, suprabasal and superficial part of the epithelium. (lower panels show optical sections through suprabasal layer).
Leukocytes in mouse corneal epithelium
The presence of the CD45 leukocytes in the mouse cornea has been described previously.13,14,15,18 By confocal microscopy it was possible to image the entire cornea and identify all leukocytes within the cornea.18 The numbers of cells expressing the pan‐leukocyte marker CD45 were similar in all regions of corneal epithelium—The mean number of cells found in the peripheral region was 124 (SD 42) per mm2, 131 (24) per mm2 in paracentral and 106 (14) per mm2 in central region.
Leukocytes in the corneal epithelium occurred in two discrete subsets. One subset (about one half of the CD45 cells) appeared to be located close to the uppermost surface of corneal epithelium, embedded within the most superficial layer of squamous epithelium (fig 5A). This subset of cells in the superficial layer had variable morphology, and many cells appeared unusually round and flat. A second population of CD45 cells was detected immediately subjacent to, or residing within, the basal layer of the corneal epithelium. These cells were more clearly defined and their cell bodies were in close contact with the epithelial cells of the basal layer (fig 5B,C). Large processes from these cells traversed the epithelial basement membrane. No CD45 leukocytes were detected in the suprabasal corneal epithelial cell layer in any of the examined corneas (fig 5D). In addition, no difference in the distribution of the two populations of leukocytes was observed with or without permeabilisation of the tissue. We assume that leukocytes are absent from this layer.
Figure 5 Leukocytes in the epithelium (A) Dual staining of corneal epithelium showimg F‐actin+ (red) cells and CD45 round leukocytes (green) within the squamous epithelium. (B,C) images showing dendritic‐like CD45 leukocytes located in the basal layer of epithelium. (D) No CD45 cells were detected in the suprabasal layer.
Distribution of tight junctional components in the mouse cornea
ZO‐1
To assess the distribution of ZO‐1 in the mouse cornea, 8 μm sections and wholemouts were prepared. In cryostat sections ZO‐1+ staining was found at the lateral margins of the most superficial cells of the epithelium as previously described.17 Some immunoreactivity was also found on the corneal endothelium (fig 6A). Wholemount corneal preparations showed ZO‐1 positivity round the entire cell border of superficial epithelial cells creating a continuous ring (fig 6B). A similar distribution was found on the endothelium (fig 6C). No ZO‐1+ was located in any other part of mouse cornea. When dual stained with phalloidin it showed that the F‐actin filaments are in intimate contact with the ZO‐1 (fig 6D).
Figure 6 Distribution of ZO‐1 in the mouse cornea (A) 8 μm section of the cornea showing immunoreactivity at the margins of the superficial cells of the epithelium and also on the endothelium. Cell nuclei are visualised by propidium iodide staining. (B) wholemount preparation of cornea showing ZO‐1 distiribution around the border of the squamous epithelial cells and (C) endothelial cells. (D) dual staining for ZO‐1 (green) and F‐actin (red) showing intimate contact in the superficial layer of the epithelium.
Claudin‐1
In mouse corneal transverse sections, we found claudin‐1 staining at cell‐cell borders of several epithelial layers particularly the suprabasal layer. No staining was detected in the stroma or endothelium (fig 7A). In wholemount corneal preparation the claudin‐1 antibody‐staining in the superficial layers showed as interrupted punctate staining and did not surround the cell in the same way as ZO‐1 staining (compare figs 6B and 7B,C). In some superficial cells, claudin‐1 staining appeared to be present throughout the cytoplasm but was interpreted as non‐specific staining in pre‐apoptotic cells (fig 7C).
Figure 7 Distribution of Claudin‐1 in the mouse cornea (A) On the 8 μm thick sections of mouse cornea, claudin‐1 immunoreactivity is present in most epithelial layers and prominently so in the suprabasal layer. No immunoreactivity was observed in the stroma or on the endothelium. (B) In wholemount preparations of cornea discontinuous punctate staining for claudin‐1 was observed around the cell borders in the superficial layer while in other cells in the superficial layers (C) intracytoplasmic loclisation of claudin‐1 could be found. Cell nuclei are visualised by propidium iodide staining.
Occludin
Staining of corneal sections showed positivity for occludin in all layers of mouse epithelium. Some immunoreactivity was also found in the endothelium (fig 8A). In whole mount preparations of cornea, occludin staining was found around the cell borders and also in the cytoplasm of the superficial cells and on the endothelium (fig 8 B,C).
Figure 8 Distribution of Occludin in the mouse cornea (A) 8 μm corneal section showed immunoreactivity for occludin in all epithelial layers and also on endothelium. (B) In corneal wholemounts occludin staining was observed around the borders of the superficial cells in the epithelium and (C) in the endothelium. Cell nuclei are visualised by propidium iodide staining.
We also examined Triton X‐100 treated corneas for tight junction immunoreactivity but the normal staining pattern for all tight junction proteins was lost after this treatment, although the overall cell morphology remained (fig 4).
Discussion
The corneal epithelium is known to be a highly effective barrier to passage of molecules.19,20 Transport systems are in place for the movements of entities as small as ions across this surface layer, accounting for the well recognised transepithelial potential difference which exists across this cell layer.21
Barrier function at epithelial and endothelial surfaces generally is attributed to a range of proteins associated with intercellular tight junctional complexes.1 For epithelial cells, and especially those of the cornea, barrier function is considered to be a property of cells in most if not all layers, as indicated by their expression of tight junction associated proteins ZO‐1 and integral junctional proteins such as occludin and claudin17,22 (figs 6–8). The barrier functional role of these proteins has been demonstrated, mostly in living cell cultures, in studies of the passage of ions and small molecules and through evidence of transepithelial electrical resistance.23
However, barrier function to the passage of molecules and cells is a matter of degree and will vary for different types of endothelium and epithelium. In particular it might be expected that the more spread squamous epithelial cell layer, despite its prominent ring of ZO‐1 staining, might be less of a barrier to the passage of molecules than the tightly packed basal corneal epithelium, or a single monolayer of cells such as the retinal pigment epithelium or the columnar respiratory epithelium. In this regard it is unclear whether there is a direct qualitative or quantitative relationship between epithelial barrier function and full expression of all integral and junctional plaque‐associated proteins. In addition, the role of individual tight junction and tight junction‐associated proteins is only now becoming clear with claudins having a major role in ion and small solute selectivity24 while the precise role of occludin remains unclear.
Most studies to date have focussed on detection of proteins in junctional complexes in simple epithelia or in monolayer cultures of epithelia. In the stratified epithelium of the skin, an effective barrier to particulate foreign materials is provided by the cornified surface keratinocytes (corneocytes25)26 which are held together by lipid lamellae secreted by the cells of the stratum granulosum. Initially opinion favoured the idea that the cornified epithelium presented the main barrier to diffusion of solutes as well as particulate material, but studies on claudin deficient mice revealed the importance of tight junctions in skin permeability where the major site of the barrier appeared to occur in the stratum granulosum.27,28 In the cornea the surface squamous cells are not cornified but have a dense covering of membrane bound mucoproteins of the mucin family which trap foreign materials and permit their removal by the sweeping movements of the eyelids.29
The data in the present study examines the location of the barrier to the passive diffusion of small molecules (phalloidin has a molecular weight of 1115 daltons and is not charged) in fixed, non living cells and thus excludes the role of active functional effects of, for instance, the actin cytoskeleton and its interaction with tight junctional complexes. The data show that the main barrier to passive diffusion in the corneal epithelium resides in the suprabasal layer, and this is where the highest density of junctional complex proteins are expressed particularly claudin which as previously stated is considered to be the main effector protein in the tight junction complex.28 Only when tight junctional complexes are disrupted by permeabilisation with detergent can small molecules passively diffuse to the deeper layers of the cornea and bind to their specific proteins.
From a physiological aspect, the presence of the barrier at the level of the suprabasal epithelial cell layer, indicates that as well as presenting a barrier to the transport of small molecules across the epithelium, this layer may also acts as the site of the well recognised immunological barrier to the trafficking of cells across the corneal layers. Thus stromal leukocytes have the potential to interpolate their cell bodies and processes into the deep basal layer of the corneal epithelium and probably have a scavenging role at this level, while surface leukocytes presumably emigrating from conjunctival vessels and other pericorneal vessels into the tears act in a similar manner to remove mucin‐trapped surface debris as part of normal ocular surface house‐keeping. It is of course still quite possible that this separation of leukocytes into tissue zones is incomplete since leukocytes themselves possess tight junction‐degrading enzymes and can rapidly traffic across epithelial layers;30 however, the complete absence of leukocytes in the suparabasal layer of the epithelium after scanning entire corneal whole mounts, and their ready detection even as occasional cells in the basal and the superficial layers suggests that the suprabasal layer may be a barrier to antero‐posterior transcorneal leukocyte traffic as well as diffusion of small molecules in the normal physiological state.
Acknowledgement
This work was supported by Development Trust of the University of Aberdeen, UK.
Abbreviations
JAM‐1 - junction adhesion molecule‐1
PBS - phosphate‐buffered saline
Footnotes
Competing interests: none.
References
- 1.Schneeberger E E, Lynch R D. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 2004286C1213–C1228. [DOI] [PubMed] [Google Scholar]
- 2.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 20012285–293. [DOI] [PubMed] [Google Scholar]
- 3.Ban Y, Dota A, Cooper L J.et al Tight junction‐related protein expression and distribution in human corneal epithelium. Exp Eye Res 200376663–669. [DOI] [PubMed] [Google Scholar]
- 4.Furuse M, Hirase T, Itoh M.et al Occludin: a novel integral membrane protein localising at tight junctions. J Cell Biol 19931231777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirokawa N, Tilney L G. Interactions between actin filaments and between actin filaments and membranes in quick‐frozen and deeply etched hair cells of the chick ear. J Cell Biol 198295249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madara J L. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol 1987253C171–C175. [DOI] [PubMed] [Google Scholar]
- 7.Wittchen E S, Haskins J, Stevenson B R. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO‐1 forms independent complexes with ZO‐2 and ZO‐3. J Biol Chem 199927435179–35185. [DOI] [PubMed] [Google Scholar]
- 8.Madara J L, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol 19861022125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madara J L, Pappenheimer J R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol 1987100149–164. [DOI] [PubMed] [Google Scholar]
- 10.Madara J L. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol 199860143–159. [DOI] [PubMed] [Google Scholar]
- 11.Itoh M, Nagafuchi A, Moroi S.et al Involvement of ZO‐1 in cadherin‐based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol 1997138181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danjo Y, Gipson I K. Actin “purse string” filaments are anchored by E‐cadherin‐mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci 19981113323–3332. [DOI] [PubMed] [Google Scholar]
- 13.Brissette‐Storkus C S, Reynolds S M, Lepisto A J.et al Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci 2002432264–2271. [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Hamrah P, Zhang Q.et al Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II‐positive dendritic cells derived from MHC class II‐negative grafts. J Exp Med 2002195259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamrah P, Zhang Q, Liu Y.et al Novel characterization of MHC class II‐negative population of resident corneal Langerhans cell‐type dendritic cells. Invest Ophthalmol Vis Sci 200243639–646. [PubMed] [Google Scholar]
- 16.Sakata M, Sack R A, Sathe S.et al Polymorphonuclear leukocyte cells and elastase in tears. Curr Eye Res 199716810–819. [DOI] [PubMed] [Google Scholar]
- 17.Yi X, Wang Y, Yu F S. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol Vis Sci 2000414093–4100. [PubMed] [Google Scholar]
- 18.Sosnova M, Bradl M, Forrester J V. CD34+ corneal stromal cells are bone marrow‐derived and express hemopoietic stem cell markers. Stem Cells 200523507–515. [DOI] [PubMed] [Google Scholar]
- 19.Klyce S D, Crosson C E. Transport processes across the rabbit corneal epithelium: a review. Curr Eye Res 19854323–331. [DOI] [PubMed] [Google Scholar]
- 20.Wolosin J M, Budak M T, Akinci M A. Ocular surface epithelial and stem cell development. Int J Dev Biol 200448981–991. [DOI] [PubMed] [Google Scholar]
- 21.Song B, Zhao M, Forrester J V.et al Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci U S A 20029913577–13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ban Y, Cooper L J, Fullwood N J.et al Comparison of ultrastructure, tight junction‐related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air‐lifting. Exp Eye Res 200376735–743. [DOI] [PubMed] [Google Scholar]
- 23.Toropainen E, Ranta V P, Talvitie A.et al Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci 2001422942–2948. [PubMed] [Google Scholar]
- 24.Schneeberger E E. Claudins form ion‐selective channels in the paracellular pathway. Focus on “Claudin extracellular domains determine paracellular charge selectively and resistance but not tight junction fibril architecture”. Am J Physiol Cell Physiol 2003284C1331–C1333. [DOI] [PubMed] [Google Scholar]
- 25.Morita K, Tsukita S, Miyachi Y. Tight junction‐associated proteins (occludin, ZO‐1, claudin‐1, claudin‐4) in squamous cell carcinoma and Bowen's disease. Br J Dermatol 2004151328–334. [DOI] [PubMed] [Google Scholar]
- 26.Morita K, Miyachi Y. Tight junctions in the skin. J Dermatol Sci 20033181–89. [DOI] [PubMed] [Google Scholar]
- 27.Brandner J M, Kief S, Grund C.et al Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol 200281253–263. [DOI] [PubMed] [Google Scholar]
- 28.Furuse M, Hata M, Furuse K.et al Claudin‐based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin‐1‐deficient mice. J Cell Biol 20021561099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gipson I K. Distribution of mucins at the ocular surface. Exp Eye Res 200478379–388. [DOI] [PubMed] [Google Scholar]
- 30.Zen K, Parkos C A. Leukocyte‐epithelial interactions. Curr Opin Cell Biol 200315557–564. [DOI] [PubMed] [Google Scholar]