Abstract
An important issue in synaptic physiology is the extent to which postsynaptic receptors are saturated by the neurotransmitter released from a single synaptic vesicle. Although the bulk of evidence supports receptor saturation, recent studies have started to reveal that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) and N-methyl-d-aspartate (NMDA) receptors may not be saturated by a single vesicle of glutamate. Here, we address this question through a study of putative single synapses, made by hippocampal neurons in culture, that are identified by FM1–43 staining. An analysis of the sources of variability in the amplitudes of miniature excitatory postsynaptic currents at single synapses reveals that this variability must arise presynaptically, from variations in the quantity of agonist released. Thus, glutamate receptors at hippocampal synapses are not generally saturated by quantal release.
One of the most fundamental issues in synaptic physiology is whether receptors at a single synapse are saturated by a quantum of neurotransmitter. In the hippocampus, fast excitatory synaptic transmission is mediated primarily by two classes of ionotropic glutamate receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) and N-methyl-d-aspartate (NMDA) receptors (AMPARs and NMDARs, respectively; ref. 1). Because the number of glutamate molecules released from a synaptic vesicle is much larger than the number of postsynaptic glutamate receptors and because the volume of the synaptic cleft is so small, it has been assumed that glutamate reaches a concentration high enough to saturate all of the postsynaptic receptors (refs. 2–4; reviewed in ref. 5). NMDARs, in particular, have been thought to be saturated because of their extremely high equilibrium affinity for glutamate (6).
The amplitudes of AMPAR-mediated miniature excitatory postsynaptic currents (mEPSCs) at one or a small number of synapses are highly variable from one spontaneous event to the next (1, 7, 8). Identifying the source of this variability can potentially reveal whether postsynaptic receptors are saturated by quantal release. Variations in mEPSC size could arise either from a presynaptic mechanism that produces fluctuations in the quantity of glutamate in the synaptic cleft, or from a postsynaptic mechanism, such as moment-to-moment fluctuations in the number of functional receptors or in their sensitivity to agonist. If the source of variability is not postsynaptic, that is, if the variability occurs because of fluctuations in the quantity of agonist in a quantum, receptors must not be saturated.
Recently, using various techniques to monitor synaptic responses at single synapses, several laboratories have confirmed that mEPSC amplitudes mediated by both AMPA and NMDARs are, indeed, highly variable, even at single synapses (9–13). Using loose-patch recording of synaptic terminals (10), local stimulation of glutamate release (11), and calcium imaging of individual dendritic spines (13), responses of AMPARs at single synapses to a single quantum of glutamate in hippocampal cultures were shown to be highly variable. Similarly, imaging calcium transients mediated by NMDARs at hippocampal synapses both in slice (12) and in culture (13) indicates that NMDAR responses to a single quantum also may be highly variable. Although these previous studies demonstrated that both AMPA and NMDA components of mEPSCs are highly variable (9–13) and possibly correlated (13), they did not establish whether the source of the fluctuations is presynaptic or postsynaptic.
Our goal here was to localize the source of variability in mEPSC amplitude to either a presynaptic or postsynaptic mechanism. We find that this variability must arise presynaptically and conclude that glutamate receptors at hippocampal synapses are, therefore, not saturated.
Methods
Hippocampal Cultures and Whole-Cell Recording.
Pyramidal neurons from the CA1/CA3 regions of the hippocampus from newborn Long-Evans rat pups were dissociated and plated onto astrocyte monolayers as described (1). Whole-cell patch-clamp recordings were made at room temperature (23–25° C) from the cell soma of pyramidal neurons. The normal external solution contained 145 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 8 mM dextrose, 10 mM Hepes (pH 7.3), plus 10 μM glycine (to enhance NMDAR opening), 1 μM tetrodotoxin (to block Na-dependent action potentials; Tocris Cookson, Baldwin, MO), 100 μM picrotoxin (to block γ-aminobutyric acid type A receptors; Research Biochemicals), 1 μM strychnine (to block glycine receptors), adjusted to 300 mOsm with sorbitol. The patch electrodes (2–4 MΩ resistance) contained a K-gluconate internal solution (130 mM K-gluconate, 10 mM KCl, 10 mM Hepes, 1 mM EGTA, 1 mM CaCl2, 2.5 mM ATP-Mg2+, 0.2 mM GTP-Li2+, pH 7.3, adjusted to 290 mOsm with sorbitol). Recordings were made under voltage clamp with an Axopatch 200 amplifier (Axon Instruments, Foster City, CA) at a holding potential of −60mV with a 2-kHz low-pass Bessel filter. Access resistance was monitored, and only cells with stable access resistance were included in the analysis. All recordings were acquired and analyzed by using custom programs written in visual basic.
Visualizing Synapses on Cultured Hippocampal Neurons.
Hippocampal cultures were stained with 10 μM FM1–43 (Molecular Probes) in external solution containing 40 mM KCl to depolarize all neurons (normal external solution as above except for 108 mM NaCl, 40 mM KCl, and no toxins). Neurons were exposed to the high [K+], FM1–43-containing solution for 1 min, then were washed thoroughly for at least 10 min to remove surface staining. The remaining punctate fluorescent spots that can be destained are synapses (14, 15). Synapses were viewed with conventional fluorescence on an inverted Olympus microscope using a 40× CDPlan lens and a Micromax cooled charge-coupled device camera from Princeton Instruments, Trenton, NJ. Images were acquired and stored with winview image acquisition software (Princeton Instruments). To enable local perfusion of single synapses, neurons were chosen for recording only if they had single synapses (single puncta) on their dendrites that were at least 5 μm from their nearest neighbors. To optimize the possibility of finding such cells, cultures were used after only 8–10 days in vitro.
Recording from Putative Single Synapses.
To record mEPSCs at single synapses, we combined whole-cell patch-clamp recording from the cell soma with local perfusion of hypertonic solution at single synapses (Fig. 1A). Hypertonic solution causes synaptic vesicles to release glutamate with similar properties as depolarization-evoked release (1, 16). After staining with 10 μM FM1–43, a neuron was patch-clamped in whole-cell mode and an external solution similar to that described above, but containing 1 μM 6-cyano-2,3-dihydroxy-7-nitroquinoxaline (CNQX) and only 0.1 mM MgCl2, was washed into the recording chamber. CNQX blocked detection of AMPAR-mediated mEPSCs from all synapses on the neuron, and low Mg2+ allowed subsequent recording of the NMDA component of each mEPSC. Once this external solution washed into the chamber, a synapse that was at least 5 μm from its neighbors was selected and two additional pipettes were positioned close to and on either side of the selected synapse (Fig. 1A; ref. 1). One of these pipettes (1 μm tip) was filled with normal external solution (as above) containing 300 mM sucrose, 0 mM Mg2+, and no CNQX. This pipette was connected to a picospritzer so that a puff of air caused the hypertonic solution to flow out of the pipette and onto the synapse. The stream of solution was immediately sucked into a larger bore (10 μm) suction pipette connected to a vacuum and placed on the opposite side of the synapse. The stream of hypertonic solution was on average 2 μm wide and was maintained for 10-sec intervals repeated every 10 sec. Recorded mEPSCs containing an AMPA component must be located at the locally perfused synapse because that is the only area where blockade of AMPARs by CNQX was removed (there was no CNQX in the hypertonic solution). We are confident that CNQX is quickly (within milliseconds) and completely removed from the activated synapse because mEPSC amplitudes do not change from the start to the end of the local stimulation. Also consistent with rapid and complete removal of CNQX, the variance measured with this method is within the same range as that measured by local stimulation with high KCl (9).
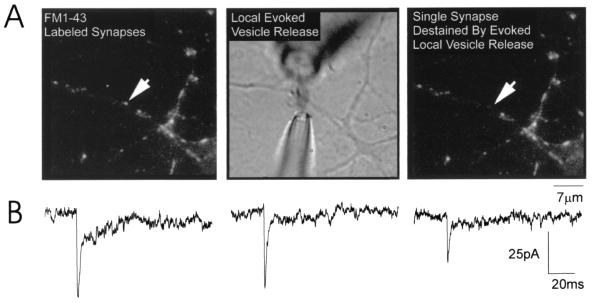
Figure 1.
Stimulation of mEPSCs at single synapses on cultured hippocampal neurons. (A) (Left) An image of the synapses on a portion of a dendrite of a hippocampal neuron maintained in vitro for 9 days. Each synapse is labeled with FM1–43; the arrow points to the single synapse from which mEPSCs were recorded. Before local perfusion, the synapse was clearly stained with FM1–43 and there was no electrical activity recorded because CNQX was present in the bath. Local perfusion was established by placing electrodes on either side of the selected synapse (Center; see Methods). Hypertonic solution containing no CNQX was puffed onto the synapse for 10 sec, every 10 sec; within milliseconds, mEPSCs were recorded. The stream was approximately 2 μm wide and never reached the neighboring synapse. The final image (Right), showing that the perfused synapse destained whereas neighboring synapses lost no fluorescence, provided confirmation that recorded mEPSCs originated exclusively from the selected synapse (arrow). (B) Representative dual-component mEPSCs recorded from a putative single synapse (cell 1 in Table 1).
Our conclusions rely on recording mEPSCs at single synapses in hippocampal cultures. Using the criteria described above, we are confident that locally evoked mEPSCs originate from a single synapse for four reasons. First, Liu and Tsien (9) previously have demonstrated that sites of single punctae of FM1–43 correspond to single synapses. Second, local perfusion elicited mEPSCs only when centered over an FM1–43-labeled synapse; moving the pipette to either side decreased and then abolished the response. Third, local perfusion also destained single synapses whereas neighboring synapses were unaffected (Fig. 1A). Finally, synapses were selected for local perfusion only if they were at least 5 μm apart from their nearest neighbors. Because the stream of hypertonic solution was only 2 μm wide, our selection criteria ensured that no other synapses were activated. It is important to note that our conclusion of single synapse stimulation also assumes a direct correspondence between single presynaptic boutons and single FM1–43-stained puncta. We consider this a valid assumption based on work from our lab and others (11, 14, 15, 17).
Discrimination of AMPA and NMDA Components of mEPSCs.
Approximately 400 mEPSCs were collected from each putative single synaptic site. To measure the NMDA component of each mEPSC accurately and to minimize effects of interactions between closely timed events, only those mEPSCs that were at least 500 msec apart were included in the analysis. Representative mEPSCs included in the analysis are shown in Fig. 1B. mEPSCs consist of two components (one fast and one slow) likely to be mediated by AMPA and NMDARs, respectively (1). These two components were completely blocked by antagonists of AMPA and NMDARs, the fast component by 1 μM CNQX and the slow component by 50 μM 2-amino-5-phosphonovaleric acid (APV). No mEPSCs were detected in the presence of both CNQX and APV (data not shown).
The amplitude of the AMPA component was measured as the peak of the fast component of the mEPSC. The amplitude of the NMDA component was calculated as follows. All mEPSCs from a single synapse (that were selected for analysis based on the criteria described above) were averaged, and an exponential was fit to the decay of the slow component, starting at a point 3× the decay time constant of the fast component after the mEPSC peak and ending 100 msec later. The decay time constant (tau) of this average exponential then was used to fit an exponential to the slow components of each individual mEPSC. Each individual exponential (for each mEPSC) was extrapolated back to the time when the mEPSC started, defining the peak NMDA amplitude.
Iontophoresis.
To estimate the contribution of postsynaptic AMPA and NMDARs to the synaptic mEPSC variability, the variance of the postsynaptic response to a fixed concentration of iontophoresed glutamate or NMDA was directly measured. Electrodes (approximately 100 MΩ) were filled with either 100 mM Na-glutamate (pH 7) or 20 mM NMDA (pH 7.3). The normal external solution described above, with 1 mM Mg2+, was used for glutamate iontophoresis to minimize NMDA currents. To measure only NMDAR-mediated responses to NMDA iontophoresis, the normal external solution was modified to contain no Mg2+ and 1 μM CNQX. A small backing current of about 1 nA was applied to prevent leakage and subsequent desensitization of glutamate receptors (reviewed in ref. 18). While recording whole-cell responses, the pipette was moved along the dendrite until a response was recorded. The pipette tip was positioned as close to the dendrite as possible without touching it. Using an SD9 stimulator (Grass Instruments, West Warwick, RI) very brief pulses of glutamate (1 msec) or NMDA (0.2 msec) then were applied every 2–4 sec and the variations in postsynaptic response from pulse to pulse at a constant stimulus strength were measured. In several experiments, after collecting responses to NMDA, 10 μM MK-801 (Research Biochemicals) was washed into the chamber with 40 mM KCl for 1 min. Because MK-801 is an open channel blocker of NMDARs and all synapses were activated by high-K stimulation, MK-801 should have blocked all NMDARs at synapses, leaving only extrasynaptic receptors unblocked (19). After thorough washout of MK-801, responses to NMDA were measured again at a variety of stimulus strengths.
Results
Both the AMPA and NMDA Components of mEPSCs at Putative Single Synapses Are Highly Variable in Amplitude.
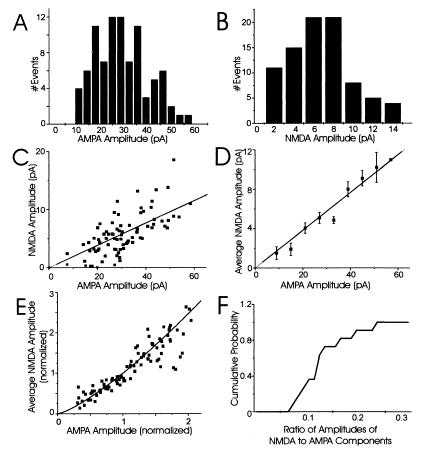
Our first objective was to confirm earlier reports that the AMPA and NMDA components of mEPSCs are highly variable in size at single synapses. To do this, we used a local perfusion technique (described above) that evokes mEPSCs locally and recorded dual-component mEPSCs from 11 single synapses (each from a separate neuron) by using whole-cell patch-clamp recording. The AMPA (fast) and NMDA (slow) components were measured and a coefficient of variation (CV = SD/mean) was calculated for each component. A large amount of variability from mEPSC to mEPSC clearly occurred at single synapses (Fig. 2 A and B; Table 1); a representative example is illustrated in Fig. 2 A and B (cell 1, Table 1). The amplitude of the AMPA and NMDA components varies within a wide range between mEPSCs at this single synapse (from 10 to 60 pA for the fast component and from 2 to 14 pA for the slow component). Consistent with previous observations (7–11, 13, 20), the CVs for the AMPA and NMDA components for this synapse are large: 0.42 and 0.65, respectively (Table 1). For all single synapses examined, the CVs of the AMPA components ranged from 0.27 to 0.43, and those for NMDA components were from 0.56 to 0.82 (Table 1). Thus, the mEPSC amplitude variability is large for both AMPA and NMDA components even at single synapses.
Figure 2.
Both AMPA and NMDA components of mEPSCs at putative single synapses are highly variable and strongly correlated. (A) Amplitude histogram of the AMPA component of all mEPSCs recorded from a single synapse (CV = 0.42; cell 1 in Table 1). (B) Amplitude histogram of the NMDA component of all mEPSCs recorded from the same single synapse (CV = 0.65). (C) A representative example of the relationship between AMPA and NMDA components at a single synapse. Each point corresponds to a single, dual-component mEPSC. The best-fit linear regression through zero is plotted for reference. (D) To determine the average correlation of the two components, the average NMDA amplitude was plotted for all mEPSCs with AMPA amplitudes within 10 bins of 6 pA (error bars are 1 SEM); the line through zero is the best-fit linear regression. The AMPA and NMDA components were highly correlated at this synapse (correlation coefficient = 0.72). (E) To compare mEPSC amplitudes from all 11 single synapses, the AMPA and NMDA components at each synapse were normalized relative to their mean amplitudes. The normalized, average NMDA amplitude for mEPSCs at each individual synapse then was plotted relative to the normalized AMPA amplitude and the best-fit linear regression constrained through zero was included for reference. (F) A cumulative probability histogram of the ratio of amplitudes of NMDA to AMPA components.
Table 1.
The average amplitudes of both AMPA and NMDA components (pA) of mEPSCs at single synapses tabulated with their corresponding CVs
| Cell # (n) | Avg. AMPA amplitude | CV of AMPA amp. | Avg. NMDA amplitude | CV of NMDA amp. | Correlation coefficient |
|---|---|---|---|---|---|
| 1 (90) | 29.6 | 0.42 | 5.6 | 0.65 | 0.72 |
| 2 (78) | 66.5 | 0.43 | 11.6 | 0.56 | 0.73 |
| 3 (58) | 30.6 | 0.38 | 3.5 | 0.59 | 0.78 |
| 4 (147) | 55.7 | 0.43 | 4.8 | 0.74 | 0.6 |
| 5 (151) | 38.1 | 0.33 | 2.6 | 0.64 | 0.66 |
| 6 (67) | 26.7 | 0.39 | 1.9 | 0.73 | 0.67 |
| 7 (112) | 32.1 | 0.31 | 4.3 | 0.62 | 0.62 |
| 8 (96) | 29.5 | 0.36 | 3 | 0.65 | 0.56 |
| 9 (109) | 23.5 | 0.37 | 3.1 | 0.66 | 0.63 |
| 10 (71) | 29.2 | 0.27 | 3.2 | 0.63 | 0.68 |
| 11 (90) | 16.3 | 0.29 | 3.2 | 0.82 | 0.6 |
n, The number of mEPSCs analyzed for each neuron. Both AMPA and NMDA components of mEPSCs at single synapses are highly variable; this result is consistent for all single synapses examined (cells 1–11). Their correlation coefficients show a strong, positive correlation between the AMPA and NMDA components for mEPSCs at all 11 single synapses.
AMPA and NMDA Components of mEPSCs at Single Synapses Are Strongly, Positively Correlated.
The next objective was to determine whether the variability in the AMPA and NMDA components of mEPSCs is independent or has a common source. If the variability arises from channel noise, the two components should not be correlated because AMPA and NMDARs are independent (21–24). Conversely, if the variability in mEPSC amplitude arises from quantum-to-quantum fluctuations in glutamate concentration in the synaptic cleft, then the variations in the two components will be highly correlated because the source of variation is common to both types of receptors. Note that a common source for fluctuations in mEPSC amplitudes could be either presynaptic or postsynaptic, and if the variability has a postsynaptic origin (e.g., fluctuations in the number of functional receptors), the existence of a common source gives no information about saturation of postsynaptic receptors.
Consistent with a common source for AMPA and NMDAR mEPSC variability, we found a clear and strong correlation between the amplitudes of the AMPA and NMDA components of mEPSCs at all 11 single synapses (Fig. 2). For example, plots of the amplitude of the AMPA components versus the NMDA components of each mEPSC show a strong, positive relationship at an individual synapse (Fig. 2C; cell 1 in Table 1). This relationship is close to linear, as demonstrated by plotting the average NMDA amplitude for a range of AMPA amplitudes (correlation coefficient = 0.72; Fig. 2D). To compare the AMPA-to-NMDA correlation for all 11 single synapses, the amplitudes of the two components were normalized relative to their means and plotted in Fig. 2E. Clearly, there is a strong, almost linear relation between the amplitudes of AMPA and NMDA components at all 11 single synapses examined; the correlation coefficient for the average AMPA and NMDA components for all single synapses was 0.91. The reason for the slight nonlinearity in this relationship is unknown, but it could reflect differences in the region of the dose-response curves sampled by each receptor type. The average amplitude of the NMDA component was, on average, 12% of the AMPA amplitude and was never greater than 30% of the AMPA amplitude (Fig. 2F). Because the stochastic gating properties of AMPA and NMDARs are so different and should not correlate (21–24), the strong, positive correlation between the AMPA and NMDA components of mEPSCs at single synapses argues against channel noise as the main source of mEPSC variability, but is consistent with either a presynaptic or postsynaptic origin of the fluctuations.
One prediction of nonsaturation of glutamate receptors is that the variance for both AMPA and NMDA components should be similar, especially if both types of receptors sense the same concentration of glutamate. However, the CVs for the NMDA component of mEPSCs are generally much larger than the corresponding CVs for the AMPA component (Table 1). Preliminary analysis indicates that measurement and channel noise greatly increase the variance of the NMDA component. Subtraction of these variances from the variance in the amplitude of the NMDA component of mEPSCs at single synapses reduces the CV for NMDAR-mediated responses into the same range as those mediated by AMPARs.
Postsynaptic Responses to Iontophoretic Application of Glutamate Show Very Little Trial-to-Trial Variability.
To estimate the magnitude of postsynaptic variability, fixed concentrations of either glutamate or NMDA were iontophoretically applied to localized sites on the postsynaptic dendrite (see Methods). Evoked responses at a number of stimulus strengths and distances from the synapse were recorded. In general, the variability in postsynaptic responsiveness of both AMPA and NMDARs to a fixed concentration of glutamate or NMDA was much less than that seen for synaptic mEPSCs.
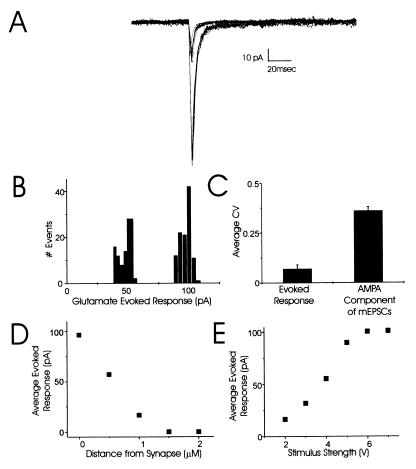
AMPAR-mediated responses to fixed concentrations of iontophoretically applied glutamate (100 mM) showed little variability (Fig. 3). For the putative single synapse described in Fig. 3, the CV was only 0.1 for 78 glutamate-evoked responses at a stimulus strength that evoked a half-maximal, 54-pA response (Fig. 3 A and B). The variability was even lower, CV = 0.04, for a maximal stimulus strength that evoked 73 responses around 100 pA (Fig. 3 A and B). Most significantly, the variability of the postsynaptic AMPARs to a fixed concentration of glutamate (CV = 0.1) was much lower than the variability of the AMPA component of mEPSCs at a single synapse (CV = 0.36; Fig. 3C). These glutamate-evoked responses are likely from a single synapse as they decrease rapidly with lateral movements of the iontophoretic pipette away from the synapse; the response is almost completely abolished with a 1-μm displacement (Fig. 3D). Finally, for all four cells examined, the amplitude of the glutamate-evoked response increased with increasing stimulus strength until it reached a plateau (100 pA for the neuron in Fig. 3E). This plateau confirms that it is possible to saturate the AMPARs at a localized site (but at currents well above those seen with mEPSCs) and that there is probably very little contribution of extrasynaptic receptors to the response.
Figure 3.
Postsynaptic responses to iontophoretic application of glutamate show very small trial-to-trial variability. (A) Families of currents at two stimulus strengths illustrate the consistency in responses to fixed glutamate concentrations. Each family contains 10 individual traces. (B) Amplitude histograms show little variability in amplitude of AMPAR-mediated responses to half-maximal (CV = 0.1) and maximal stimulus strengths (CV = 0.04) for glutamate iontophoresis. (C) Pooled data for the CV of currents evoked by glutamate iontophoresis (CV = 0.1; four separate experiments) compared with the CV for the AMPA component of mEPSCs recorded at single synapses (CV = 0.36; average of all 11 synapses). (D) Glutamate-evoked responses decrease rapidly with lateral movements of the iontophoretic pipette away from the synapse; the response is almost completely abolished with a 1-μm displacement. (E). The maximal amplitude of the average evoked response (pA) to increasing concentrations of iontophoretically applied glutamate at this synapse plateaued at approximately 100 pA.
Postsynaptic Responses to Iontophoretic Application of NMDA Also Show Very Little Trial-to-Trial Variability.
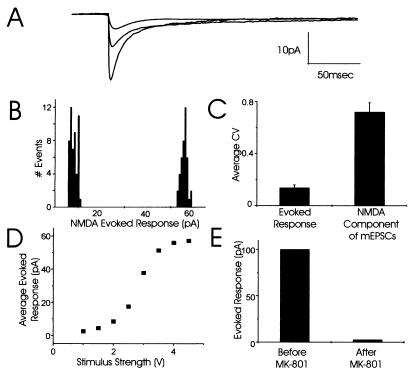
NMDARs responded with similar properties as AMPARs to fixed concentrations of agonist (Fig. 4). Responses to iontophoresis of a fixed concentration of NMDA showed very little trial-to-trial variability at all stimulus strengths tested (Fig. 4A). For example, the CV for responses at the minimal stimulus strength that evoked an 8.4-pA response on average (52 responses) was 0.18 and the CV for responses at the maximal stimulus strength that evoked a 51.4-pA response on average (41 responses) was only 0.06 (Fig. 4B). Clearly, the variability of the postsynaptic NMDARs to a fixed concentration of NMDA (CV = 0.18) was much lower than the variability of the NMDA component of mEPSCs at single synapses (CV = 0.73; Fig. 4C). Like AMPARs, the responses of NMDARs to NMDA iontophoresis could be saturated and reached a clear plateau (Fig. 4D). However, it was also possible to recruit additional NMDARs by increasing the stimulus strength 10-fold above the strength that evoked the plateau response. This observation suggests that either extrasynaptic or synaptic receptors from nearby synapses can be recruited with extremely high concentrations of NMDA. Nevertheless, these results support the conclusion that NMDARs at these synapses are not saturated as the plateau response to iontophoresed NMDA (60 pA) was much larger than even the largest NMDA component of mEPSCs at single synapses (11.6 pA; Table 1).
Figure 4.
Postsynaptic responses to iontophoretic application of NMDA show very little trial-to-trial variability. (A) Families of currents at three stimulus strengths illustrate the consistency of responses to fixed NMDA concentrations. Each family contains three individual traces. (B) Amplitude histograms show little variability in amplitude of NMDAR-mediated responses to minimal and maximal stimulus strengths for NMDA iontophoresis. (C) Pooled data for the CV of currents evoked by NMDA iontophoresis (CV = 0.18; three separate experiments) compared with the CV for the NMDA component of mEPSCs recorded at single synapses (CV = 0.73; average of all 11 synapses). (D) The maximal amplitude of the average evoked response (pA) to increasing concentrations of iontophoretically applied NMDA at this synapse plateaued at approximately 60 pA. (E) At a separate synapse, the average evoked response to NMDA iontophoresis was approximately 100 pA and was completely abolished by selectively blocking synaptic NMDARs with the open channel blocker, MK-801.
The NMDAR Iontophoretic Responses Arise from Synaptic, Not Extrasynaptic, Receptors.
Because glutamate receptors are distributed extrasynaptically as well as being concentrated at the synapse (19), our iontophoretically induced responses might have been dominated by extrasynaptic rather than by synaptic receptors. To examine this possibility, we first measured localized responses to NMDA iontophoresis, then blocked all synaptic NMDARs with the open-channel blocker, MK-801, and then again recorded responses to NMDA iontophoresis at the site where synaptic NMDARs were blocked. By comparing the amplitude of the response to iontophoretically applied NMDA before and after blocking the synaptic receptors, we could determine the relative contributions of synaptic and extrasynaptic receptors to our recorded responses. MK-801 was applied in the presence of 40 mM KCl to stimulate glutamate release at all synapses, which leads to most of the NMDARs opening and thus allows MK-801 to block all synaptic NMDARs. Synaptic NMDARs mediated most of the evoked responses to iontophoresis, as these responses were almost completely abolished by MK-801 (Fig. 4E). Interestingly, increasing the iontophoretic current 10-fold above the stimulus level used before MK-801 block revealed clear evoked responses, consistent with the presence of extrasynaptic NMDARs (19).
These results from both glutamate and NMDA iontophoresis demonstrate that the high mEPSC variability at single synapses does not arise from variability in postsynaptic receptor responsiveness. Moreover, as the largest mEPSC amplitude is smaller than the maximal response to iontophoresed agonist, these results provide further support for nonsaturation of both AMPA and NMDARs at single hippocampal synapses in culture. Thus, the variability in mEPSC amplitude at single synapses must be caused by a presynaptic mechanism that results in fluctuations in the amount of glutamate in the synaptic cleft.
Discussion
Because the number of glutamate molecules released from a synaptic vesicle into the very small volume of the synaptic cleft far exceeds the number of postsynaptic receptors, it generally has been assumed that glutamate receptors at excitatory synapses in the central nervous system are saturated by quantal release (refs. 2–4; reviewed in ref. 5). To the contrary, we demonstrate here that neither AMPA nor NMDARs at hippocampal synapses in culture are saturated by release of a single vesicle of glutamate. We make this assertion based on the following observations: (i) The AMPA and NMDA components of mEPSCs at single synapses are highly variable (Fig. 2). (ii) The two components are also tightly correlated (Fig. 2), demonstrating that a common mechanism accounts for their variability in amplitude, and excluding channel noise as the mechanism for this variability. (iii) Fluctuations in postsynaptic sensitivity to agonist contributes very little to the variability in mEPSC amplitude at single synapses, as the variability of AMPA and NMDAR responses to iontophoretic application of fixed concentrations of glutamate is quite low (Figs. 3 and 4). Thus, the variability of mEPSC amplitudes at single synapses must be a function of fluctuations in the concentration of glutamate in the synaptic cleft after each neurotransmitter release event and, therefore, neither AMPA nor NMDARs are saturated by a single quantum of glutamate.
The classic conclusion that glutamate receptors are saturated by quantal release relies on estimates of the glutamate concentration in a synaptic vesicle, the volume of each vesicle, the dimensions of the synaptic cleft, and the equilibrium dissociation constants of AMPA and NMDARs (refs. 2–4; reviewed in ref. 5). These estimates convincingly predict that the instantaneous rise in glutamate concentration near postsynaptic glutamate receptors after vesicular release must be greater than the dissociation constants from AMPA and NMDARs. However, these models also predict that the maximum fraction of receptors that binds glutamate in the estimated range of peak glutamate concentrations is sensitive to the peak concentration and precise time course of glutamate in the synaptic cleft (ref. 2; reviewed in ref. 5). The glutamate concentration in the synaptic cleft will be dramatically reduced both by binding to glutamate transporters (25, 26) and by diffusion out of the cleft (ref. 27; reviewed in ref. 5). It is possible that clearance of glutamate from the synaptic cleft is so fast that a significant fraction of AMPA and NMDARs remains unbound for most mEPSCs (5, 27–29).
Although several papers addressing the issue of mEPSC amplitude variability at single synapses recently have been published (8–13), none of those studies has localized the source of this variability for both AMPA and NMDA components at a single synapse. Because AMPARs have a lower equilibrium affinity for glutamate than NMDARs, it has been assumed that AMPARs may not be saturated by a single quantum of glutamate whereas NMDARs are very likely to be saturated (ref. 6; reviewed in ref. 5). Indeed, it was elegantly demonstrated that the variability in mEPSC amplitude mediated by AMPARs at putative single hippocampal synapses is caused by fluctuations in the glutamate concentration in the synaptic cleft (11). Similarly, it is also likely that the large variability in γ-aminobutyric acid (GABA) receptor-mediated miniature inhibitory postsynaptic current amplitude in amacrine cells is caused by fluctuations in the amount of GABA in each quantum, although it remains formally possible that changes in the numbers or properties of GABA receptors could contribute (30). Recently, two additional laboratories have reported large fluctuations in the amount of NMDAR-mediated calcium influx from release to release and have provided compelling evidence that NMDARs on hippocampal neurons are also unlikely to be saturated by quantal release (12, 13). However, those studies do not address the source of the variability in amplitude of the NMDA component of mEPSCs. Here, we directly demonstrate that both AMPA and NMDARs are not saturated by a quantum of glutamate and that the source of the large variability in mEPSC amplitude at single hippocampal synapses is the result of a presynaptic mechanism.
Because the response of postsynaptic glutamate receptors to agonist exhibits little variability, the large variability in mEPSC amplitude must arise from fluctuations in the concentration of glutamate in the synaptic cleft. There are several possible sources for this variability in cleft glutamate concentration. First, the amount of glutamate packaged in each vesicle may vary substantially because of variations in the volume of synaptic vesicles (7). Based on an estimated CV of synaptic vesicle diameters of 11–13%, synaptic vesicle volumes at hippocampal synapses may vary by 40% (7, 31). The amount of glutamate packaged in single vesicles will vary by the third power of the vesicle diameter (7, 30). Second, clearance of glutamate from the synaptic cleft may vary from release to release (refs. 25 and 26; reviewed in ref. 5). Finally, variations in the site of exocytosis of synaptic vesicles relative to the location of postsynaptic glutamate receptors could cause variable amounts of glutamate to be sensed by postsynaptic receptors (32, 33).
Resolving the issues of postsynaptic glutamate receptor saturation and mechanisms of quantal variability at central synapses is critical for understanding the elementary unit of synaptic transmission and its modulation during synaptic plasticity (reviewed in ref. 34). Our results demonstrate that the mEPSC amplitude at single synapses is modulated by the transient glutamate concentration in the synaptic cleft, rather than by rapid changes in the number of functional glutamate receptors. Changes in vesicular packaging, release, or uptake therefore may contribute to determining the amplitude and variability of postsynaptic responses, and hence may modulate plasticity. As NMDAR activation is critical for plasticity of synaptic transmission at many central neurons, the degree of NMDAR activation may determine whether a synapse undergoes long-term depression or potentiation (reviewed in ref. 35). Thus, synaptic strength may be significantly influenced by changes in the concentration of glutamate in the synaptic cleft, independent of possible changes in release probability or numbers of postsynaptic receptors.
Acknowledgments
We thank Jane Sullivan and Jeff Isaacson for insightful comments on this manuscript and Richard Jacobs for technical assistance. This work was supported by the Howard Hughes Medical Institute (C.F.S.).
Abbreviations
- CV
coefficient of variation
- mEPSC
miniature excitatory postsynaptic current
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- AMPAR
AMPA receptor
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
- CNQX
6-cyano-2,3-dihydroxy-7-nitroquinoxaline
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100126497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100126497
References
- 1.Bekkers J M, Stevens C F. Nature (London) 1989;341:230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- 2.Clements J, Lester R, Tong G, Westbrook G. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 3.Hestrin S. Neuron. 1992;9:991–999. doi: 10.1016/0896-6273(92)90250-h. [DOI] [PubMed] [Google Scholar]
- 4.Nusser Z, Lujan R, Laube G, Roberts J D, Molnar E, Somogyi P. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 5.Frerking M, Wilson M. Curr Opin Neurobiol. 1996;6:395–403. doi: 10.1016/s0959-4388(96)80125-5. [DOI] [PubMed] [Google Scholar]
- 6.Patneau D K, Mayer M L. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekkers J M, Richerson G, Stevens C F. Proc Natl Acad Sci USA. 1990;87:5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekkers J M, Stevens C F. J Neurophysiol. 1995;73:1145–1156. doi: 10.1152/jn.1995.73.3.1145. [DOI] [PubMed] [Google Scholar]
- 9.Liu G, Tsien R J. Nature (London) 1995;375:404–408. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- 10.Forti L, Bossi M, Bergamaschi A, Villa A, Malgaroli A. Nature (London) 1997;388:874–878. doi: 10.1038/42251. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Choi S, Tsien R J. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 12.Mainen Z F, Malinow R, Svoboda K. Nature (London) 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- 13.Umemiya M, Senda M, Murphy T H. J Physiol (London) 1999;521:113–122. doi: 10.1111/j.1469-7793.1999.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan T A, Reuter H, Wendland B, Schweizer F E, Tsien R W, Smith S J. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- 15.Murthy V N, Sejnowski T J, Stevens C F. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 16.Rosenmund C, Stevens C F. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 17.Schikorski T, Stevens C F. J Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dingledine R, Borges K, Bowie D, Traynelis S. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 19.Rosenmund C, Feltz A, Westbrook G L. J Neurosci. 1995;15:2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raastad M, Storm J F, Andersen P. Eur J Neurosci. 1992;4:113–117. doi: 10.1111/j.1460-9568.1992.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 21.Faber D S, Young W S, Legendre P, Korn H. Science. 1991;258:1494–1498. doi: 10.1126/science.1279813. [DOI] [PubMed] [Google Scholar]
- 22.Wahl L M, Pouzat C, Stratford K J. J Neurophysiol. 1996;75:597–608. doi: 10.1152/jn.1996.75.2.597. [DOI] [PubMed] [Google Scholar]
- 23.Colquhoun D, Jonas P, Sakmann B. J Physiol (London) 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spruston N, Jonas P, Sakmann B. J Physiol (London) 1995;482:325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond J S, Jahr C E. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong G, Jahr C E. Neuron. 1994;13:1159–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 27.Holmes W R. Biophys J. 1995;69:1734–1747. doi: 10.1016/S0006-3495(95)80043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silver R A, Cull-Candy S G, Takahashi T. J Physiol (London) 1996;494:231–250. doi: 10.1113/jphysiol.1996.sp021487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullmann D M. Nature (London) 1999;399:111–112. doi: 10.1038/20089. [DOI] [PubMed] [Google Scholar]
- 30.Frerking M, Borges S, Wilson M. Neuron. 1995;15:885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 31.Palay S L, Chan-Palay V. J Microsc. 1973;97:41–47. doi: 10.1111/j.1365-2818.1973.tb03759.x. [DOI] [PubMed] [Google Scholar]
- 32.Edwards F A, Konnerth A, Sakmann B. J Physiol (London) 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogt K, Luscher H-R, Streit J. Eur J Physiol. 1995;430:1022–1028. doi: 10.1007/BF01837420. [DOI] [PubMed] [Google Scholar]
- 34.Stevens C F. Cell Suppl. 1993;72:55–63. doi: 10.1016/s0092-8674(05)80028-5. [DOI] [PubMed] [Google Scholar]
- 35.Linden D J. Neuron. 1999;22:661–666. doi: 10.1016/s0896-6273(00)80726-6. [DOI] [PubMed] [Google Scholar]