Abstract
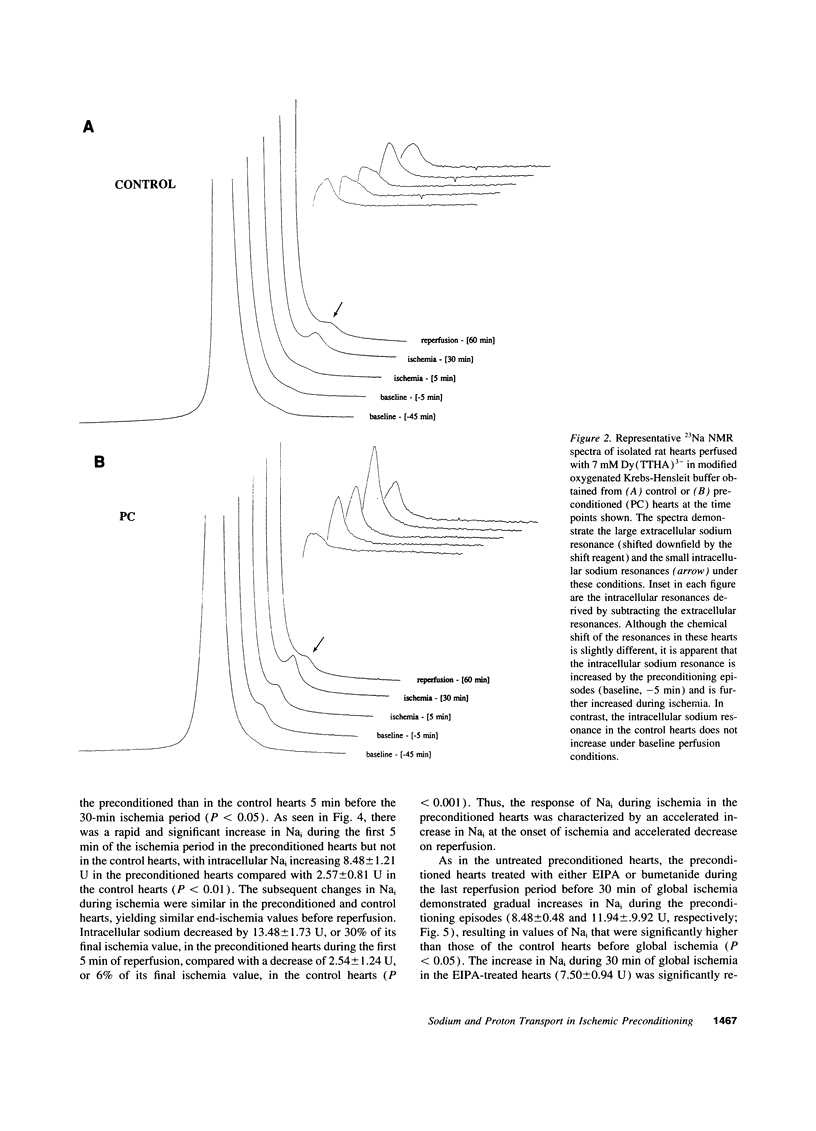
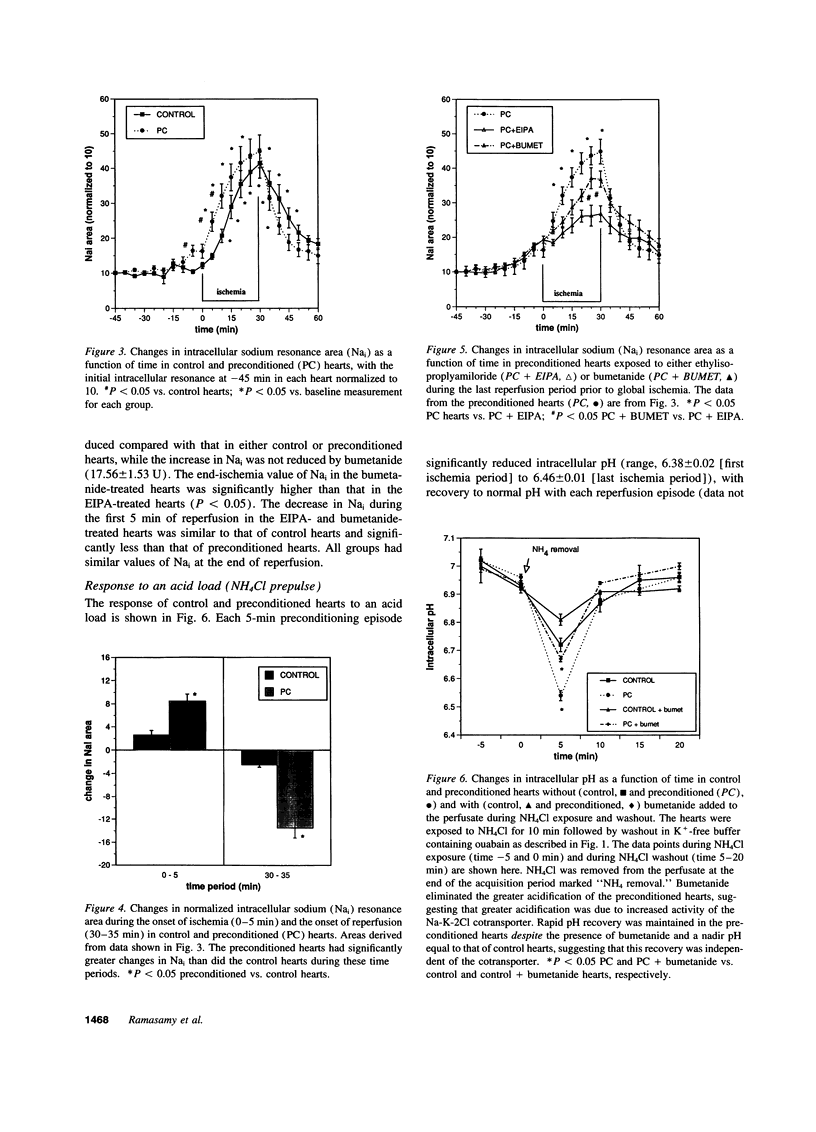
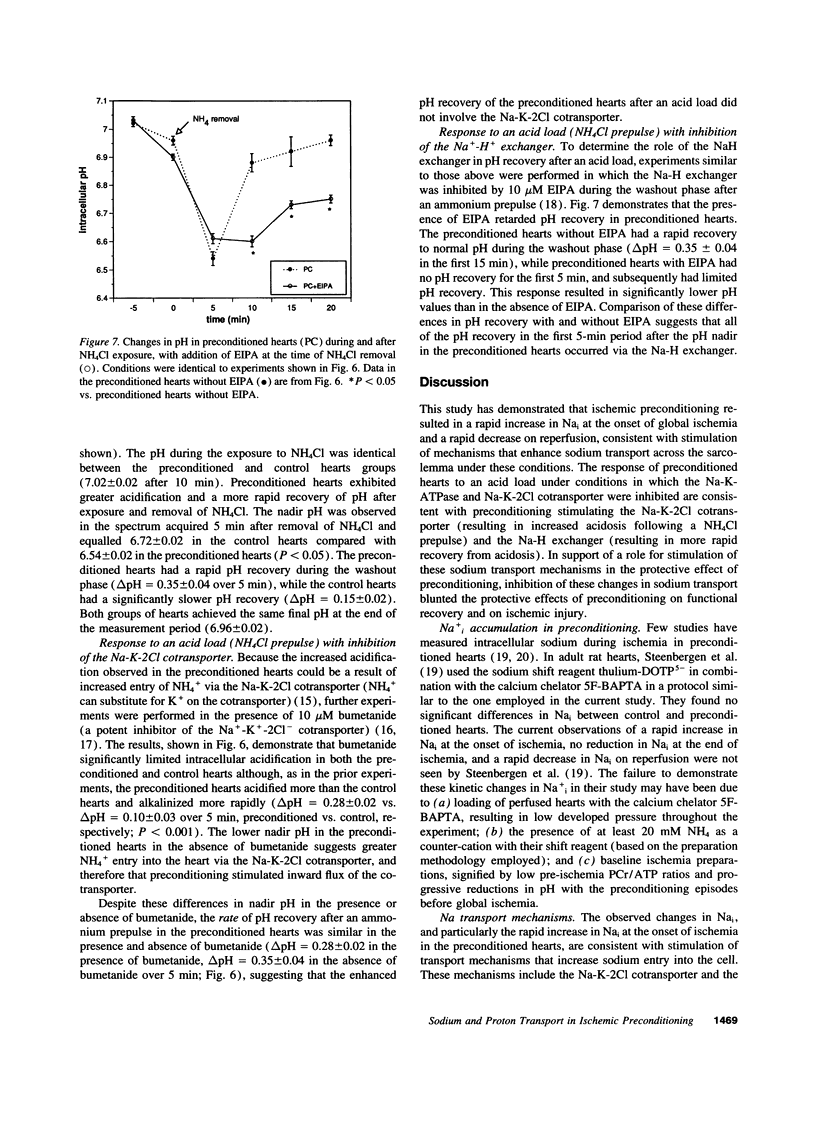
One or more brief periods of ischemia, termed preconditioning, dramatically limits infarct size and reduces intracellular acidosis during subsequent ischemia, potentially via enhanced sarcolemmal proton efflux mechanisms. To test the hypothesis that preconditioning increases the functional activity of sodium-dependent proton efflux pathways, isolated rat hearts were subjected to 30 min of global ischemia with or without preconditioning. Intracellular sodium (Nai) was assessed using 23Na magnetic resonance spectroscopy, and the activity of the Na-H exchanger and Na-K-2Cl cotransporter was measured by transiently exposing the hearts to an acid load (NH4Cl washout). Creatine kinase release was reduced by greater than 60% in the preconditioned hearts (P < 0.05) and was associated with improved functional recovery on reperfusion. Preconditioning increased Nai by 6.24 +/- 2.04 U, resulting in a significantly higher level of Nai before ischemia than in the control hearts. Nai increased significantly at the onset of ischemia (8.48 +/- 1.21 vs. 2.57 +/- 0.81 U, preconditioned vs. control hearts; P < 0.01). Preconditioning did not reduce Nai accumulation during ischemia, but the decline in Nai during the first 5 min of reperfusion was significantly greater in the preconditioned than in the control hearts (13.48 +/- 1.73 vs. 2.54 +/- 0.41 U; P < 0.001). Exposure of preconditioned hearts to ethylisopropylamiloride or bumetanide in the last reperfusion period limited in the increase in Nai during ischemia and reduced the beneficial effects of preconditioning. After the NH4Cl prepulse, preconditioned hearts acidified significantly more than control hearts and had significantly more rapid recovery of pH (preconditioned, delta pH = 0.35 +/- 0.04 U over 5 min; control, delta pH = 0.15 +/- 0.02 U over 5 min). This rapid pH recovery was not affected by inhibition of the Na-K-2Cl cotransporter but was abolished by inhibition of the Na-H exchanger. These results demonstrate that preconditioning alters the kinetics of Nai accumulation during global ischemia as well as proton transport after NH4Cl washout. These observations are consistent with stimulation of the Na-K-2Cl cotransporter and Na-H exchanger by preconditioning.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buser P. T., Wikman-Coffelt J., Wu S. T., Derugin N., Parmley W. W., Higgins C. B. Postischemic recovery of mechanical performance and energy metabolism in the presence of left ventricular hypertrophy. A 31P-MRS study. Circ Res. 1990 Mar;66(3):735–746. doi: 10.1161/01.res.66.3.735. [DOI] [PubMed] [Google Scholar]
- Cala P. M., Maldonado H. M. pH regulatory Na/H exchange by Amphiuma red blood cells. J Gen Physiol. 1994 Jun;103(6):1035–1053. doi: 10.1085/jgp.103.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowska K., Baumgarten C. M. Regulation of cellular volume in rabbit ventricular myocytes: bumetanide, chlorothiazide, and ouabain. Am J Physiol. 1991 Jan;260(1 Pt 1):C122–C131. doi: 10.1152/ajpcell.1991.260.1.C122. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Kirschenlohr H. L., Metcalfe J. C., Smith G. A., Weissberg P. L., Cragoe E. J., Jr, Vandenberg J. I. Regulation of intracellular pH in the perfused heart by external HCO3- and Na(+)-H+ exchange. Am J Physiol. 1993 Jul;265(1 Pt 2):H289–H298. doi: 10.1152/ajpheart.1993.265.1.H289. [DOI] [PubMed] [Google Scholar]
- Gross G. J., Auchampach J. A. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992 Feb;70(2):223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- Grover G. J., Sleph P. G., Dzwonczyk S. Role of myocardial ATP-sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1-receptors. Circulation. 1992 Oct;86(4):1310–1316. doi: 10.1161/01.cir.86.4.1310. [DOI] [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Igarashi P., Freed M. I., Ganz M. B., Reilly R. F. Effects of chronic metabolic acidosis on Na(+)-H+ exchangers in LLC-PK1 renal epithelial cells. Am J Physiol. 1992 Jul;263(1 Pt 2):F83–F88. doi: 10.1152/ajprenal.1992.263.1.F83. [DOI] [PubMed] [Google Scholar]
- Kida M., Fujiwara H., Ishida M., Kawai C., Ohura M., Miura I., Yabuuchi Y. Ischemic preconditioning preserves creatine phosphate and intracellular pH. Circulation. 1991 Dec;84(6):2495–2503. doi: 10.1161/01.cir.84.6.2495. [DOI] [PubMed] [Google Scholar]
- Klein J. D., Perry P. B., O'Neill W. C. Regulation by cell volume of Na(+)-K(+)-2Cl- cotransport in vascular endothelial cells: role of protein phosphorylation. J Membr Biol. 1993 Mar;132(3):243–252. doi: 10.1007/BF00235741. [DOI] [PubMed] [Google Scholar]
- Kost G. J. pH standardization for phosphorus-31 magnetic resonance heart spectroscopy at different temperatures. Magn Reson Med. 1990 Jun;14(3):496–506. doi: 10.1002/mrm.1910140307. [DOI] [PubMed] [Google Scholar]
- Li G. C., Vasquez J. A., Gallagher K. P., Lucchesi B. R. Myocardial protection with preconditioning. Circulation. 1990 Aug;82(2):609–619. doi: 10.1161/01.cir.82.2.609. [DOI] [PubMed] [Google Scholar]
- Liu G. S., Thornton J., Van Winkle D. M., Stanley A. W., Olsson R. A., Downey J. M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991 Jul;84(1):350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ytrehus K., Downey J. M. Evidence that translocation of protein kinase C is a key event during ischemic preconditioning of rabbit myocardium. J Mol Cell Cardiol. 1994 May;26(5):661–668. doi: 10.1006/jmcc.1994.1078. [DOI] [PubMed] [Google Scholar]
- Lytle C., Forbush B., 3rd The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem. 1992 Dec 15;267(35):25438–25443. [PubMed] [Google Scholar]
- Mitani A., Shattock M. J. Role of Na-activated K channel, Na-K-Cl cotransport, and Na-K pump in [K]e changes during ischemia in rat heart. Am J Physiol. 1992 Aug;263(2 Pt 2):H333–H340. doi: 10.1152/ajpheart.1992.263.2.H333. [DOI] [PubMed] [Google Scholar]
- Murry C. E., Richard V. J., Reimer K. A., Jennings R. B. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990 Apr;66(4):913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. E. Endothelial cell sodium-potassium-chloride cotransport. Evidence of regulation by Ca2+ and protein kinase C. J Biol Chem. 1991 Jun 25;266(18):11559–11566. [PubMed] [Google Scholar]
- O'Donnell M. E., Owen N. E. Sodium cotransport in vascular smooth muscle cells. Blood Vessels. 1991;28(1-3):138–146. doi: 10.1159/000158853. [DOI] [PubMed] [Google Scholar]
- O'Grady S. M., Palfrey H. C., Field M. Characteristics and functions of Na-K-Cl cotransport in epithelial tissues. Am J Physiol. 1987 Aug;253(2 Pt 1):C177–C192. doi: 10.1152/ajpcell.1987.253.2.C177. [DOI] [PubMed] [Google Scholar]
- Pewitt E. B., Hegde R. S., Haas M., Palfrey H. C. The regulation of Na/K/2Cl cotransport and bumetanide binding in avian erythrocytes by protein phosphorylation and dephosphorylation. Effects of kinase inhibitors and okadaic acid. J Biol Chem. 1990 Dec 5;265(34):20747–20756. [PubMed] [Google Scholar]
- Pike M. M., Luo C. S., Clark M. D., Kirk K. A., Kitakaze M., Madden M. C., Cragoe E. J., Jr, Pohost G. M. NMR measurements of Na+ and cellular energy in ischemic rat heart: role of Na(+)-H+ exchange. Am J Physiol. 1993 Dec;265(6 Pt 2):H2017–H2026. doi: 10.1152/ajpheart.1993.265.6.H2017. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Shigeto N., Horres C. R., Lieberman M. Na/H exchange in cultured chick heart cells: secondary stimulation of electrogenic transport during recovery from intracellular acidosis. J Mol Cell Cardiol. 1986 Nov;18(11):1109–1116. doi: 10.1016/s0022-2828(86)80036-0. [DOI] [PubMed] [Google Scholar]
- Ramasamy R., Zhao P., Gitomer W. L., Sherry A. D., Malloy C. R. Determination of chloride potential in perfused rat hearts by nuclear magnetic resonance spectroscopy. Am J Physiol. 1992 Dec;263(6 Pt 2):H1958–H1962. doi: 10.1152/ajpheart.1992.263.6.H1958. [DOI] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Rubin Y., Navon G. Inhibition of sodium influx and improved preservation of rat hearts during hypothermic ischemia by furosemide and bumetanide: a 23Na- and 31P-NMR study. J Mol Cell Cardiol. 1993 Dec;25(12):1403–1411. doi: 10.1006/jmcc.1993.1157. [DOI] [PubMed] [Google Scholar]
- Saxena R., Saksa B. A., Fields A. P., Ganz M. B. Activation of Na/H exchanger in mesangial cells is associated with translocation of PKC isoforms. Am J Physiol. 1993 Jul;265(1 Pt 2):F53–F60. doi: 10.1152/ajprenal.1993.265.1.F53. [DOI] [PubMed] [Google Scholar]
- Schaefer S., Carr L. J., Kreutzer U., Jue T. Myocardial adaptation during acute hibernation: mechanisms of phosphocreatine recovery. Cardiovasc Res. 1993 Nov;27(11):2044–2051. doi: 10.1093/cvr/27.11.2044. [DOI] [PubMed] [Google Scholar]
- Schaefer S., Carr L. J., Prussel E., Ramasamy R. Effects of glycogen depletion on ischemic injury in isolated rat hearts: insights into preconditioning. Am J Physiol. 1995 Mar;268(3 Pt 2):H935–H944. doi: 10.1152/ajpheart.1995.268.3.H935. [DOI] [PubMed] [Google Scholar]
- Scholz W., Albus U., Linz W., Martorana P., Lang H. J., Schölkens B. A. Effects of Na+/H+ exchange inhibitors in cardiac ischemia. J Mol Cell Cardiol. 1992 Jul;24(7):731–739. doi: 10.1016/0022-2828(92)93387-y. [DOI] [PubMed] [Google Scholar]
- Schott R. J., Rohmann S., Braun E. R., Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res. 1990 Apr;66(4):1133–1142. doi: 10.1161/01.res.66.4.1133. [DOI] [PubMed] [Google Scholar]
- Shell W. E., Kjekshus J. K., Sobel B. E. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest. 1971 Dec;50(12):2614–2625. doi: 10.1172/JCI106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen C., Perlman M. E., London R. E., Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res. 1993 Jan;72(1):112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- Tani M., Neely J. R. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res. 1989 Oct;65(4):1045–1056. doi: 10.1161/01.res.65.4.1045. [DOI] [PubMed] [Google Scholar]
- Wallert M. A., Fröhlich O. Alpha 1-adrenergic stimulation of Na-H exchange in cardiac myocytes. Am J Physiol. 1992 Nov;263(5 Pt 1):C1096–C1102. doi: 10.1152/ajpcell.1992.263.5.C1096. [DOI] [PubMed] [Google Scholar]