Abstract
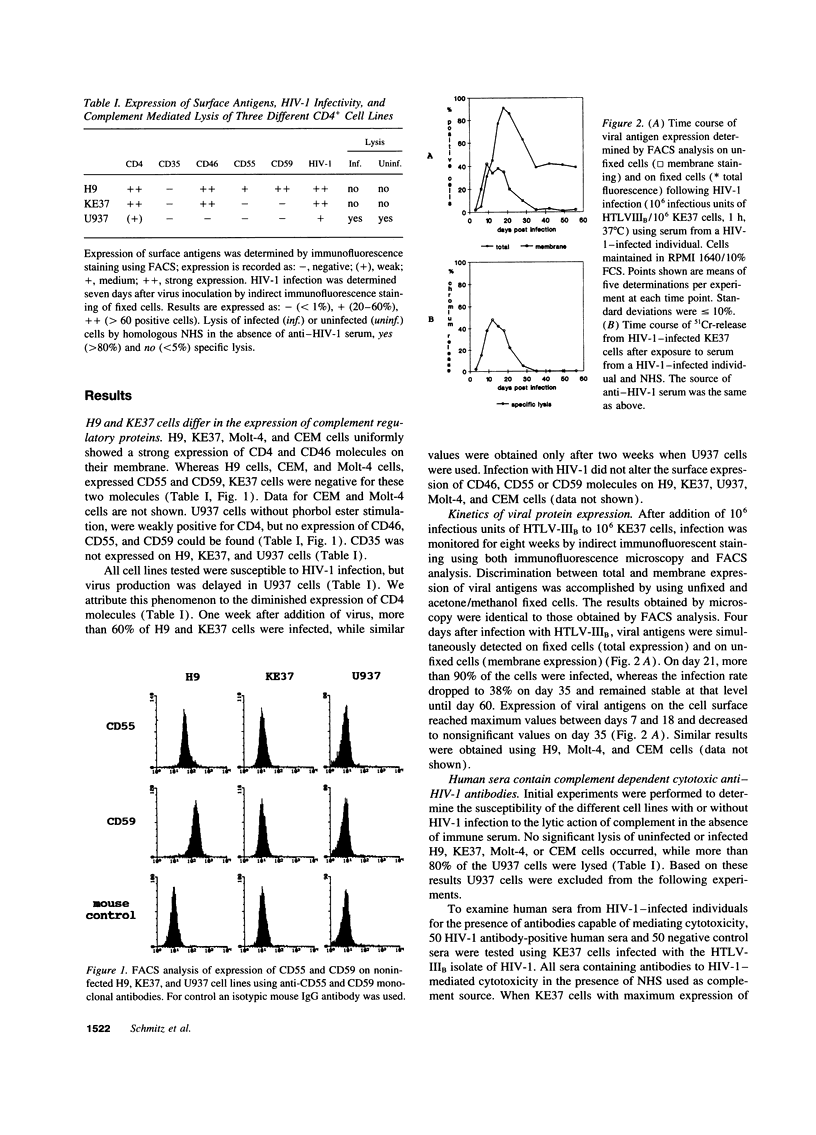
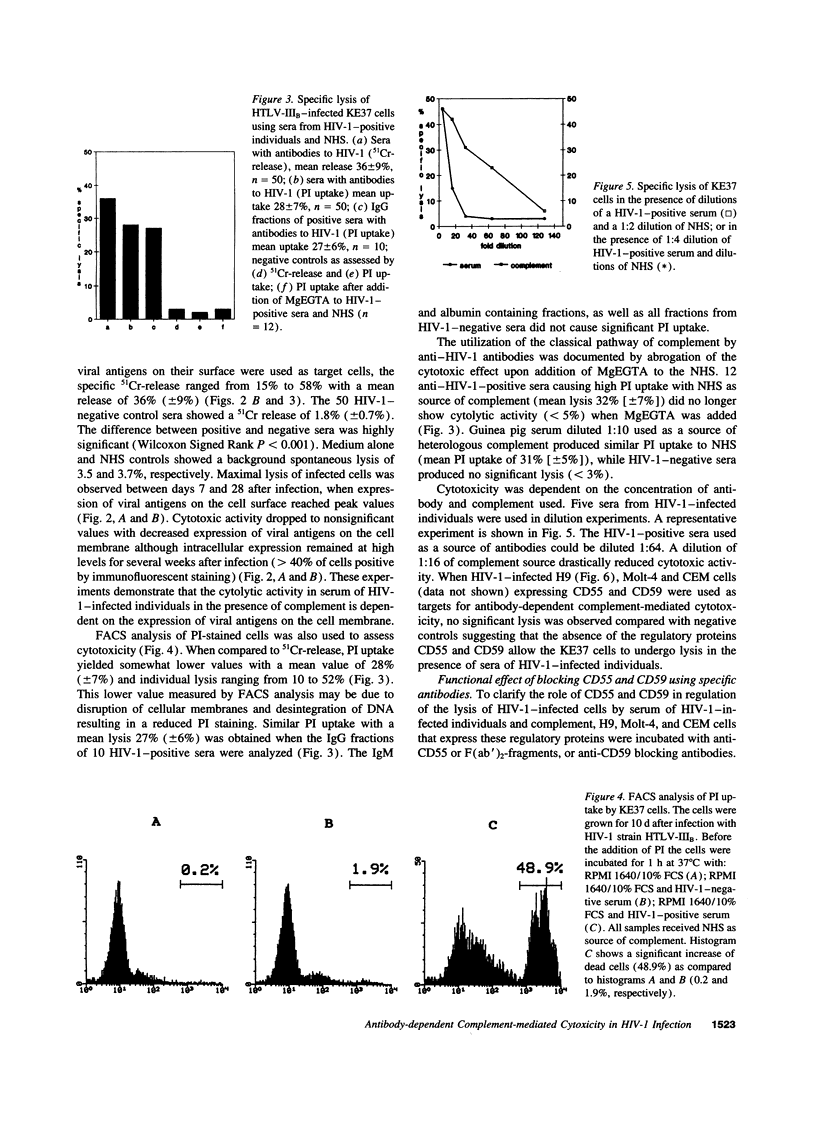
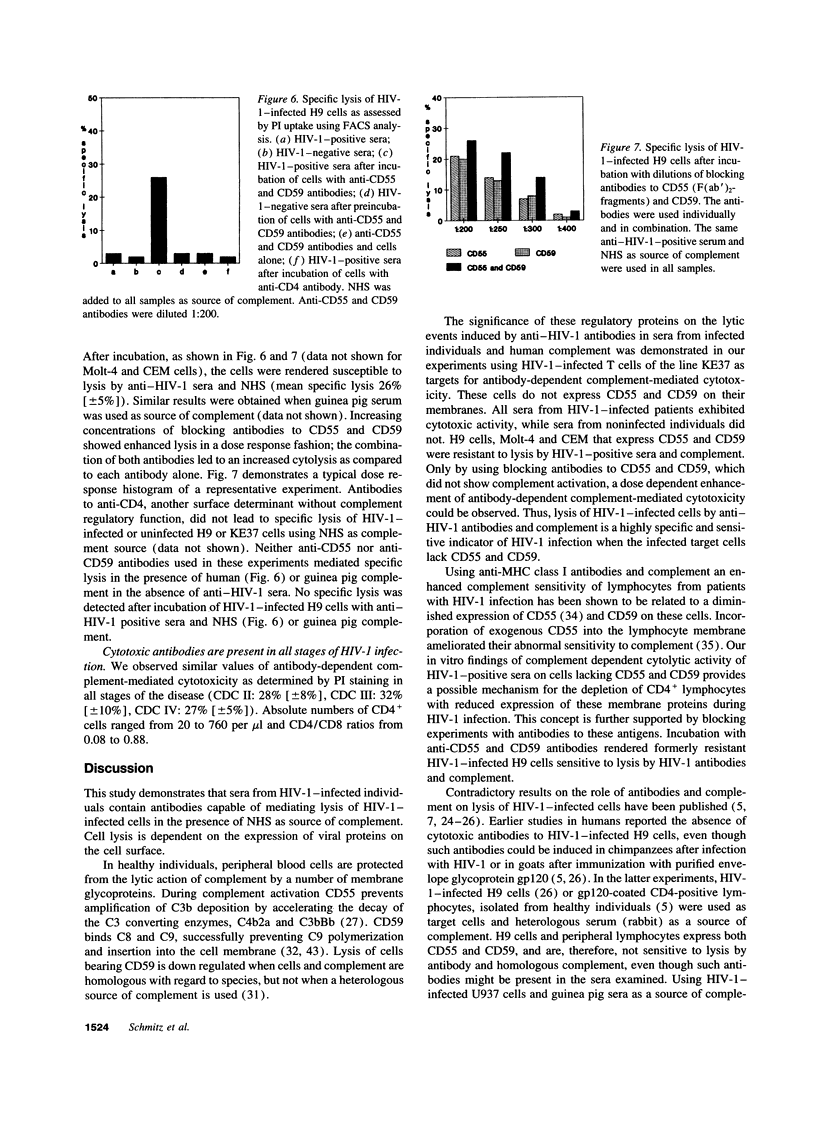
Various immune mechanisms have been reported to contribute to the progressive destruction of Th cells in HIV-1-infected patients. Among these, complement mediated lysis of infected cells has been suggested. An increased sensitivity of lymphocytes from HIV-1-infected patients to lysis by monoclonal antibodies directed to MHC class I antigen and complement has been directly correlated with a decreased expression of the decay accelerating factor (CD55). It also has been reported that the expression of the membrane inhibitor of reactive lysis (CD59) is decreased during HIV-1 infection. We examined the effect of antibodies in the serum of HIV-1-positive individuals and normal human serum (NHS) as source of complement on several HIV-1-infected cell lines differing in their expression of CD55 and CD59. When HIV-1-infected target cells without membrane expression of CD55 and CD59 were used, a highly significant cytotoxic effect was observed in the presence of heat inactivated anti-HIV-1-positive sera and NHS, while heat-inactivated anti-HIV-1-negative sera and NHS were unable to induce cytolysis. Similar results were obtained using purified IgG isolated from HIV-1-positive sera and either NHS or guinea pig serum as source of complement. Lysis of HIV-1-infected cells correlated with expression of viral antigens on the cell surface. HIV-1-infected CD55 and CD59 positive target cells showed specific lysis, when the function of these molecules was abrogated by blocking antibodies to CD55 and CD59. The finding of anti-HIV-1-specific cytotoxic antibodies in sera from HIV-1-infected patients should be considered in the pathogenesis of the HIV-1-infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banapour B., Sernatinger J., Levy J. A. The AIDS-associated retrovirus is not sensitive to lysis or inactivation by human serum. Virology. 1986 Jul 15;152(1):268–271. doi: 10.1016/0042-6822(86)90392-2. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bartholomew R. M., Esser A. F., Müller-Eberhard H. J. Lysis of oncornaviruses by human serum. Isolation of the viral complement (C1) receptor and identification as p15E. J Exp Med. 1978 Mar 1;147(3):844–853. doi: 10.1084/jem.147.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broliden P. A., von Gegerfelt A., Clapham P., Rosen J., Fenyö E. M., Wahren B., Broliden K. Identification of human neutralization-inducing regions of the human immunodeficiency virus type 1 envelope glycoproteins. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):461–465. doi: 10.1073/pnas.89.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Jensen F. C., Welsh R. M., Jr, Oldstone M. B. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976 Oct 1;144(4):970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierich M. P., Ebenbichler C. F., Marschang P., Füst G., Thielens N. M., Arlaud G. J. HIV and human complement: mechanisms of interaction and biological implication. Immunol Today. 1993 Sep;14(9):435–440. doi: 10.1016/0167-5699(93)90246-H. [DOI] [PubMed] [Google Scholar]
- Ebenbichler C. F., Thielens N. M., Vornhagen R., Marschang P., Arlaud G. J., Dierich M. P. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J Exp Med. 1991 Dec 1;174(6):1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen J. P., Mehdi S., Baur A., Hilfenhaus J. Antibody- and complement-mediated lysis of HIV-infected cells and inhibition of viral replication. J Med Virol. 1990 Apr;30(4):287–293. doi: 10.1002/jmv.1890300411. [DOI] [PubMed] [Google Scholar]
- Hamelmann C., Foerster B., Burchard G. D., Horstmann R. D. Lysis of pathogenic and nonpathogenic Entamoeba histolytica by human complement: methodological analysis. Parasite Immunol. 1992 Jan;14(1):23–35. doi: 10.1111/j.1365-3024.1992.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Holguin M. H., Fredrick L. R., Bernshaw N. J., Wilcox L. A., Parker C. J. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989 Jul;84(1):7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima A., Iwata K., Seya T., Matsumoto M., Ariga H., Atkinson J. P., Nagasawa S. Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol. 1993 Aug 1;151(3):1519–1527. [PubMed] [Google Scholar]
- Lachmann P. J. The control of homologous lysis. Immunol Today. 1991 Sep;12(9):312–315. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- Lederman M. M., Purvis S. F., Walter E. I., Carey J. T., Medof M. E. Heightened complement sensitivity of acquired immunodeficiency syndrome lymphocytes related to diminished expression of decay-accelerating factor. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4205–4209. doi: 10.1073/pnas.86.11.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr H. A., Zimmer J. P., Hübner C., Ballmann M., Hachmann W., Vogel W., Baisch H., Hartter P., Albani M., Kohlschütter A. Decreased binding of HIV-1 and vasoactive intestinal peptide following plasma membrane fluidization of CD4+ cells by phenytoin. Virology. 1990 Dec;179(2):609–617. doi: 10.1016/0042-6822(90)90128-e. [DOI] [PubMed] [Google Scholar]
- Lyerly H. K., Matthews T. J., Langlois A. J., Bolognesi D. P., Weinhold K. J. Human T-cell lymphotropic virus IIIB glycoprotein (gp120) bound to CD4 determinants on normal lymphocytes and expressed by infected cells serves as target for immune attack. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4601–4605. doi: 10.1073/pnas.84.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Middeldorp J. M., Jongsma J., The T. H. Killing of human cytomegalovirus-infected fibroblasts by antiviral antibody and complement. J Infect Dis. 1986 Jan;153(1):48–55. doi: 10.1093/infdis/153.1.48. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Robey W. G., Gonda M. A., Carter S. G., Fischinger P. J. Absence of cytotoxic antibody to human immunodeficiency virus-infected cells in humans and its induction in animals after infection or immunization with purified envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3797–3801. doi: 10.1073/pnas.84.11.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Nishanian P., Keith D. E., Jr, Houghton R. L., Heitjan D. F., Fahey J. L., Giorgi J. V. Antibodies to human immunodeficiency virus in human sera induce cell-mediated lysis of human immunodeficiency virus-infected cells. J Immunol. 1987 Oct 1;139(7):2458–2463. [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1(2):205–208. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K. Differences between the binding sites of the complement regulatory proteins DAF, CR1, and factor H on C3 convertases. J Immunol. 1986 Mar 15;136(6):2216–2221. [PubMed] [Google Scholar]
- Patrick Sissons J. G., Schreiber R. D., Perrin L. H., Cooper N. R., Müller-Eberhard H. J., Oldstone M. B. Lysis of measles virus-infected cells by the purified cytolytic alternative complement pathway and antibody. J Exp Med. 1979 Sep 19;150(3):445–454. doi: 10.1084/jem.150.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. R., Elboim H. S., Cannon T., Cavacini L., Hideshima T. Functional activity of an HIV-1 neutralizing IgG human monoclonal antibody: ADCC and complement-mediated lysis. AIDS Res Hum Retroviruses. 1992 May;8(5):553–558. doi: 10.1089/aid.1992.8.553. [DOI] [PubMed] [Google Scholar]
- Ratnoff W. D., Knez J. J., Prince G. M., Okada H., Lachmann P. J., Medof M. E. Structural properties of the glycoplasmanylinositol anchor phospholipid of the complement membrane attack complex inhibitor CD59. Clin Exp Immunol. 1992 Mar;87(3):415–421. doi: 10.1111/j.1365-2249.1992.tb03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger E. C., Vogetseder W., Berzow D., Köfler D., Bitterlich G., Lehr H. A., Wachter H., Dierich M. P. Complement-mediated enhancement of HIV-1 infection of the monoblastoid cell line U937. AIDS. 1990 Oct;4(10):961–965. doi: 10.1097/00002030-199010000-00003. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Arthur L. O., Matthews T. J., Langlois A., Copeland T. D., Lerche N. W., Oroszlan S., Bolognesi D. P., Gilden R. V., Fischinger P. J. Prospect for prevention of human immunodeficiency virus infection: purified 120-kDa envelope glycoprotein induces neutralizing antibody. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7023–7027. doi: 10.1073/pnas.83.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Lane H. C., Folks T., McCoy S., Alter H., Fauci A. S. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol. 1987 Feb 15;138(4):1064–1067. [PubMed] [Google Scholar]
- Rooney I. A., Davies A., Morgan B. P. Membrane attack complex (MAC)-mediated damage to spermatozoa: protection of the cells by the presence on their membranes of MAC inhibitory proteins. Immunology. 1992 Mar;75(3):499–506. [PMC free article] [PubMed] [Google Scholar]
- Spear G. T. Interaction of non-antibody factors with HIV in plasma. AIDS. 1993 Sep;7(9):1149–1157. doi: 10.1097/00002030-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Spear G. T., Jiang H. X., Sullivan B. L., Gewurz H., Landay A. L., Lint T. F. Direct binding of complement component C1q to human immunodeficiency virus (HIV) and human T lymphotrophic virus-I (HTLV-I) coinfected cells. AIDS Res Hum Retroviruses. 1991 Jul;7(7):579–585. doi: 10.1089/aid.1991.7.579. [DOI] [PubMed] [Google Scholar]
- Spear G. T., Landay A. L., Sullivan B. L., Dittel B., Lint T. F. Activation of complement on the surface of cells infected by human immunodeficiency virus. J Immunol. 1990 Feb 15;144(4):1490–1496. [PubMed] [Google Scholar]
- Spear G. T., Sullivan B. L., Landay A. L., Lint T. F. Neutralization of human immunodeficiency virus type 1 by complement occurs by viral lysis. J Virol. 1990 Dec;64(12):5869–5873. doi: 10.1128/jvi.64.12.5869-5873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear G. T., Takefman D. M., Sullivan B. L., Landay A. L., Zolla-Pazner S. Complement activation by human monoclonal antibodies to human immunodeficiency virus. J Virol. 1993 Jan;67(1):53–59. doi: 10.1128/jvi.67.1.53-59.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabò B., Locardi C., Lo Presti E., Belardelli F., Benedetto A. Tumour necrosis factor-alpha increases the sensitivity of human immunodeficiency virus (HIV)-infected monocytic U937 cells to the complement-dependent cytotoxicity of sera from HIV type 1-infected individuals; role of the gp120 protein. J Gen Virol. 1993 Jul;74(Pt 7):1271–1276. doi: 10.1099/0022-1317-74-7-1271. [DOI] [PubMed] [Google Scholar]
- Sölder B. M., Schulz T. F., Hengster P., Löwer J., Larcher C., Bitterlich G., Kurth R., Wachter H., Dierich M. P. HIV and HIV-infected cells differentially activate the human complement system independent of antibody. Immunol Lett. 1989 Aug;22(2):135–145. doi: 10.1016/0165-2478(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Süsal C., Kirschfink M., Kröpelin M., Daniel V., Opelz G. Complement activation by recombinant HIV-1 glycoprotein gp120. J Immunol. 1994 Jun 15;152(12):6028–6034. [PubMed] [Google Scholar]
- Tausk F. A., McCutchan A., Spechko P., Schreiber R. D., Gigli I. Altered erythrocyte C3b receptor expression, immune complexes, and complement activation in homosexual men in varying risk groups for acquired immune deficiency syndrome. J Clin Invest. 1986 Oct;78(4):977–982. doi: 10.1172/JCI112688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausk F., Fey M., Gigli I. Endocytosis and shedding of the decay accelerating factor on human polymorphonuclear cells. J Immunol. 1989 Nov 15;143(10):3295–3302. [PubMed] [Google Scholar]
- Thieblemont N., Haeffner-Cavaillon N., Weiss L., Maillet F., Kazatchkine M. D. Complement activation by gp160 glycoprotein of HIV-1. AIDS Res Hum Retroviruses. 1993 Mar;9(3):229–233. doi: 10.1089/aid.1993.9.229. [DOI] [PubMed] [Google Scholar]
- Weiss L., Okada N., Haeffner-Cavaillon N., Hattori T., Faucher C., Kazatchkine M. D., Okada H. Decreased expression of the membrane inhibitor of complement-mediated cytolysis CD59 on T-lymphocytes of HIV-infected patients. AIDS. 1992 Apr;6(4):379–385. doi: 10.1097/00002030-199204000-00004. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Cooper N. R., Jensen F. C., Oldstone M. B. Human serum lyses RNA tumour viruses. Nature. 1975 Oct 16;257(5527):612–614. doi: 10.1038/257612a0. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Wood L. M., Müller-Eberhard H. J. Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6975–6979. doi: 10.1073/pnas.83.18.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg C. W., Williams O. M., Morgan B. P. Presence of a dysfunctional form of CD59 on a CD59+ subclone of the U937 cell line. Immunology. 1994 Apr;81(4):637–642. [PMC free article] [PubMed] [Google Scholar]


