Abstract
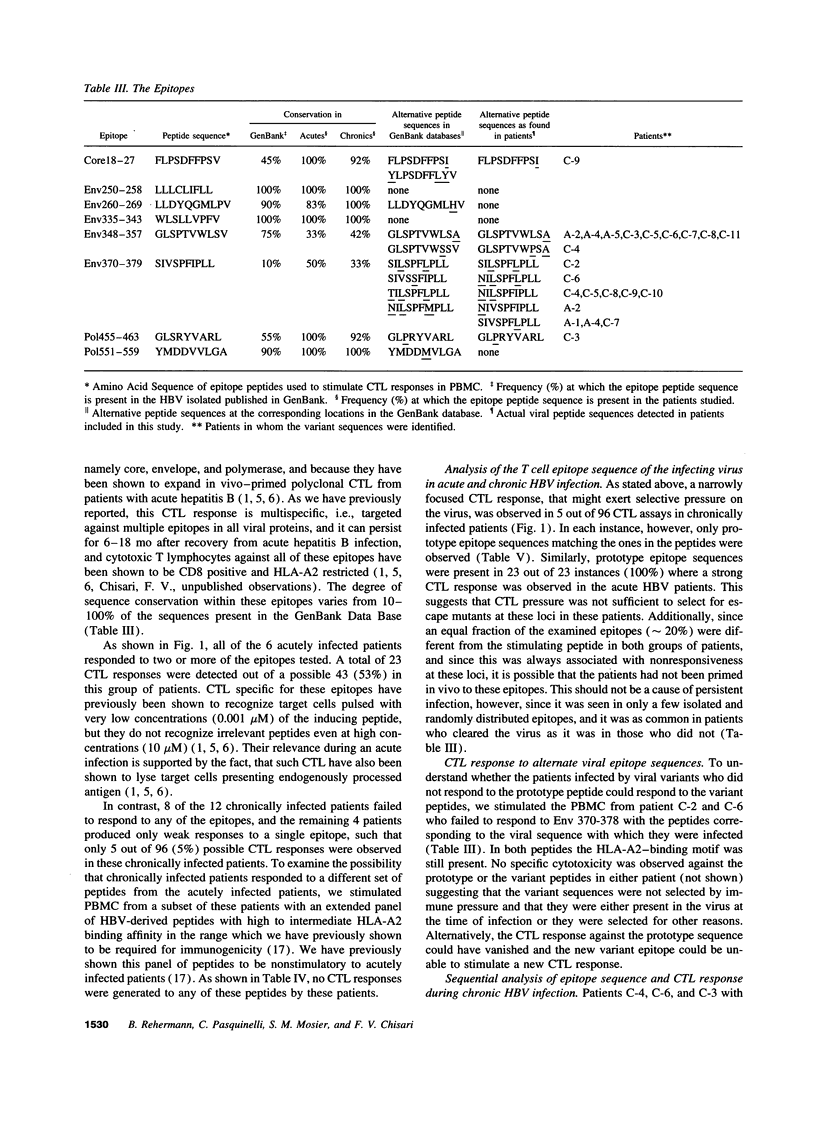
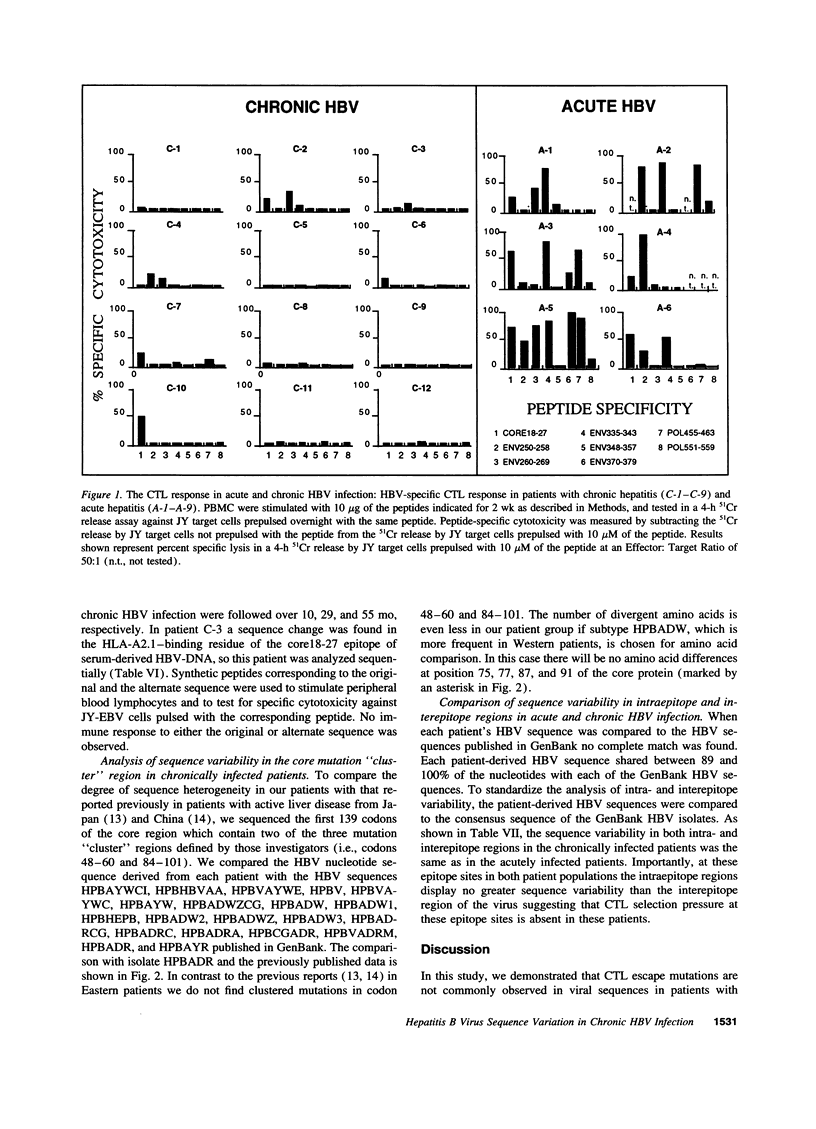
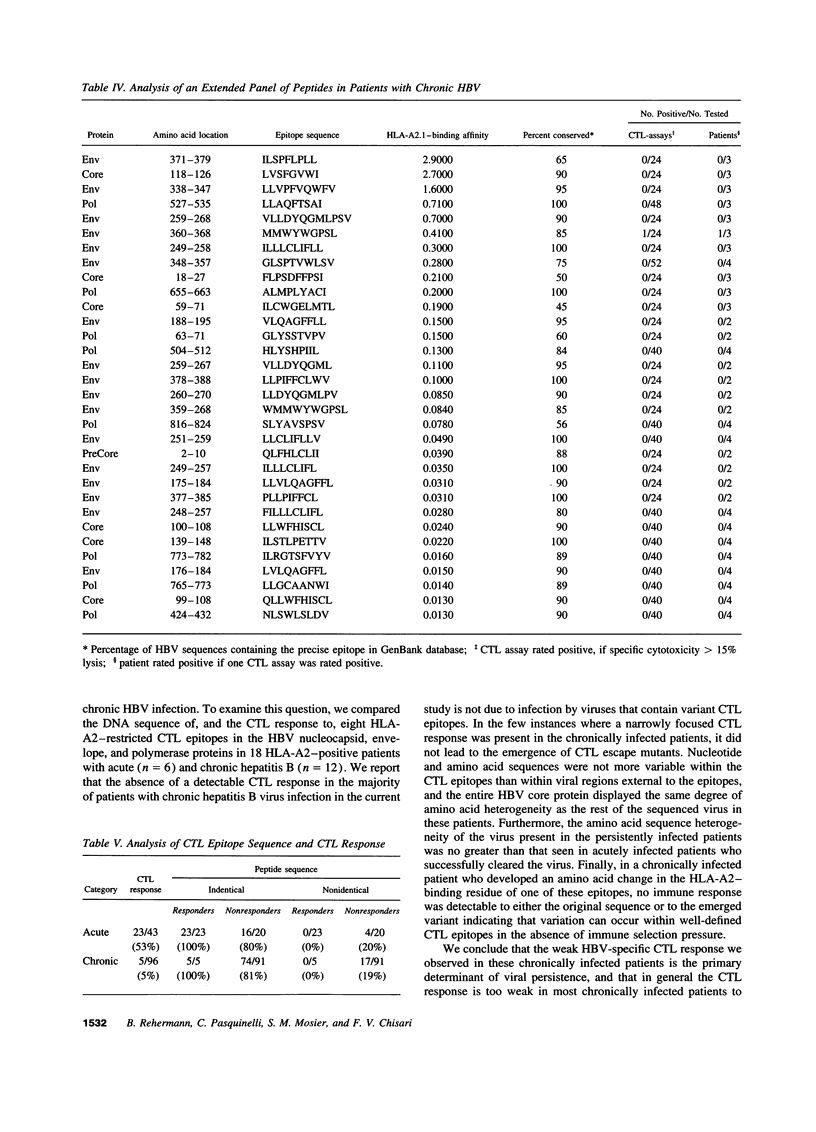
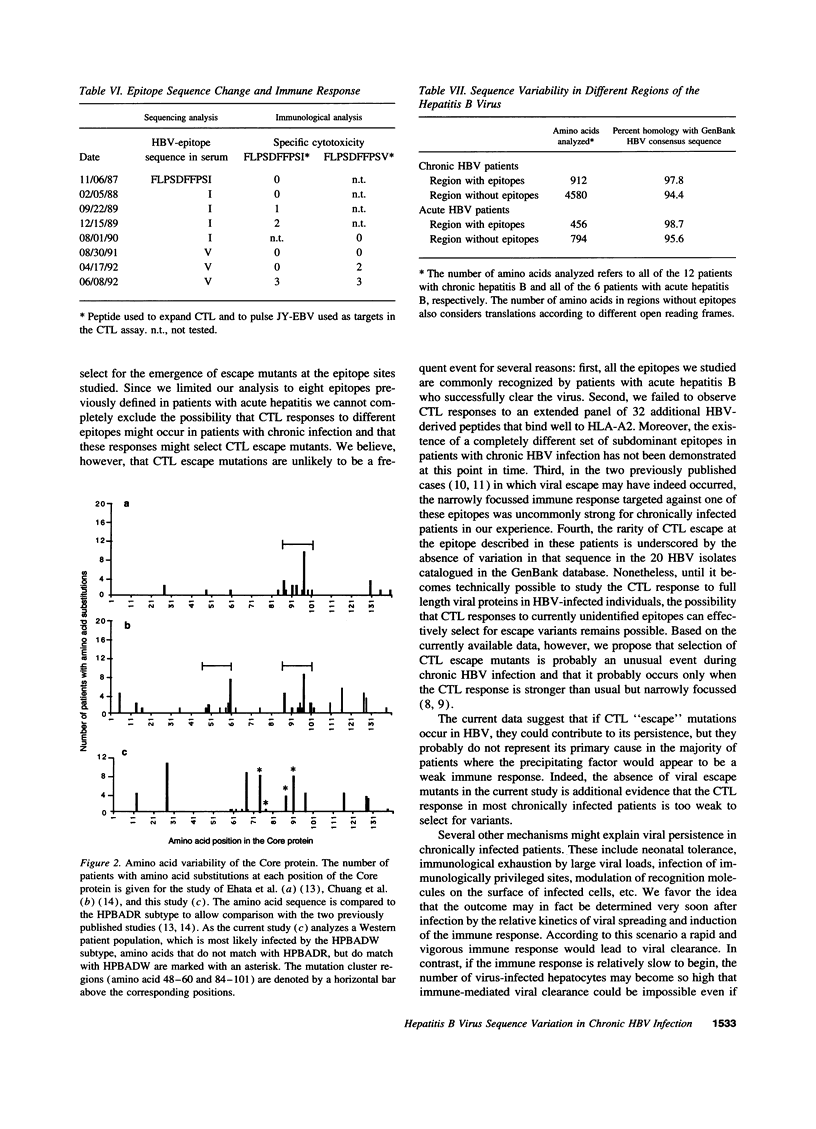
It has been suggested that immune selection pressure exerted by the cytotoxic T lymphocyte (CTL) response could be responsible for viral persistence during chronic hepatitis B virus infection. To address this question, in the current study we compared the DNA and amino acid sequences of, and the CTL responses to, multiple HLA-A2-restricted CTL epitopes in the hepatitis B virus in several HLA-A2-positive patients with acute and chronic hepatitis. Our results indicate that the CTL response to these epitopes is barely detectable in the majority of patients with chronic hepatitis. Further, we show that the weak CTL response is not secondary in infection by mutant viruses lacking these epitopes, and we show that the CTL response did not select for escape mutants in any of these patients. We conclude that an ineffective hepatitis B virus specific CTL response is the primary determinant of viral persistence in chronic hepatitis and that immune selection of viral variants is not a common event in the majority of patients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnaba V., Franco A., Alberti A., Balsano C., Benvenuto R., Balsano F. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989 Oct 15;143(8):2650–2655. [PubMed] [Google Scholar]
- Bertoletti A., Chisari F. V., Penna A., Guilhot S., Galati L., Missale G., Fowler P., Schlicht H. J., Vitiello A., Chesnut R. C. Definition of a minimal optimal cytotoxic T-cell epitope within the hepatitis B virus nucleocapsid protein. J Virol. 1993 Apr;67(4):2376–2380. doi: 10.1128/jvi.67.4.2376-2380.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A., Costanzo A., Chisari F. V., Levrero M., Artini M., Sette A., Penna A., Giuberti T., Fiaccadori F., Ferrari C. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J Exp Med. 1994 Sep 1;180(3):933–943. doi: 10.1084/jem.180.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A., Ferrari C., Fiaccadori F., Penna A., Margolskee R., Schlicht H. J., Fowler P., Guilhot S., Chisari F. V. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10445–10449. doi: 10.1073/pnas.88.23.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A., Sette A., Chisari F. V., Penna A., Levrero M., De Carli M., Fiaccadori F., Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994 Jun 2;369(6479):407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- Chuang W. L., Omata M., Ehata T., Yokosuka O., Ito Y., Imazeki F., Lu S. N., Chang W. Y., Ohto M. Precore mutations and core clustering mutations in chronic hepatitis B virus infection. Gastroenterology. 1993 Jan;104(1):263–271. doi: 10.1016/0016-5085(93)90861-6. [DOI] [PubMed] [Google Scholar]
- Ehata T., Omata M., Chuang W. L., Yokosuka O., Ito Y., Hosoda K., Ohto M. Mutations in core nucleotide sequence of hepatitis B virus correlate with fulminant and severe hepatitis. J Clin Invest. 1993 Mar;91(3):1206–1213. doi: 10.1172/JCI116281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C., Penna A., Giuberti T., Tong M. J., Ribera E., Fiaccadori F., Chisari F. V. Intrahepatic, nucleocapsid antigen-specific T cells in chronic active hepatitis B. J Immunol. 1987 Sep 15;139(6):2050–2058. [PubMed] [Google Scholar]
- Michalak T. I., Pasquinelli C., Guilhot S., Chisari F. V. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest. 1994 Jan;93(1):230–239. doi: 10.1172/JCI116950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale G., Redeker A., Person J., Fowler P., Guilhot S., Schlicht H. J., Ferrari C., Chisari F. V. HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J Exp Med. 1993 Mar 1;177(3):751–762. doi: 10.1084/jem.177.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayersina R., Fowler P., Guilhot S., Missale G., Cerny A., Schlicht H. J., Vitiello A., Chesnut R., Person J. L., Redeker A. G. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993 May 15;150(10):4659–4671. [PubMed] [Google Scholar]
- Penna A., Chisari F. V., Bertoletti A., Missale G., Fowler P., Giuberti T., Fiaccadori F., Ferrari C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991 Dec 1;174(6):1565–1570. doi: 10.1084/jem.174.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R. M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990 Aug 16;346(6285):629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Raimondo G., Stemler M., Schneider R., Wildner G., Squadrito G., Will H. Latency and reactivation of a precore mutant hepatitis B virus in a chronically infected patient. J Hepatol. 1990 Nov;11(3):374–380. doi: 10.1016/0168-8278(90)90224-f. [DOI] [PubMed] [Google Scholar]
- Rehermann B., Fowler P., Sidney J., Person J., Redeker A., Brown M., Moss B., Sette A., Chisari F. V. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995 Mar 1;181(3):1047–1058. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Vitiello A., Reherman B., Fowler P., Nayersina R., Kast W. M., Melief C. J., Oseroff C., Yuan L., Ruppert J. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994 Dec 15;153(12):5586–5592. [PubMed] [Google Scholar]
- Wakita T., Kakumu S., Shibata M., Yoshioka K., Ito Y., Shinagawa T., Ishikawa T., Takayanagi M., Morishima T. Detection of pre-C and core region mutants of hepatitis B virus in chronic hepatitis B virus carriers. J Clin Invest. 1991 Dec;88(6):1793–1801. doi: 10.1172/JCI115500. [DOI] [PMC free article] [PubMed] [Google Scholar]



