Abstract
Objective To evaluate the effect of oral decontamination on the incidence of ventilator associated pneumonia and mortality in mechanically ventilated adults.
Design Systematic review and meta-analysis.
Data sources Medline, Embase, CINAHL, the Cochrane Library, trials registers, reference lists, conference proceedings, and investigators in the specialty.
Review methods Two independent reviewers screened studies for inclusion, assessed trial quality, and extracted data. Eligible trials were randomised controlled trials enrolling mechanically ventilated adults that compared the effects of daily oral application of antibiotics or antiseptics with no prophylaxis.
Results 11 trials totalling 3242 patients met the inclusion criteria. Among four trials with 1098 patients, oral application of antibiotics did not significantly reduce the incidence of ventilator associated pneumonia (relative risk 0.69, 95% confidence interval 0.41 to 1.18). In seven trials with 2144 patients, however, oral application of antiseptics significantly reduced the incidence of ventilator associated pneumonia (0.56, 0.39 to 0.81). When the results of the 11 trials were pooled, rates of ventilator associated pneumonia were lower among patients receiving either method of oral decontamination (0.61, 0.45 to 0.82). Mortality was not influenced by prophylaxis with either antibiotics (0.94, 0.73 to 1.21) or antiseptics (0.96, 0.69 to 1.33) nor was duration of mechanical ventilation or stay in the intensive care unit.
Conclusions Oral decontamination of mechanically ventilated adults using antiseptics is associated with a lower risk of ventilator associated pneumonia. Neither antiseptic nor antibiotic oral decontamination reduced mortality or duration of mechanical ventilation or stay in the intensive care unit.
Introduction
Ventilator associated pneumonia remains a leading cause of morbidity and mortality among mechanically ventilated patients, with the incidence ranging from 9% to 27% and a crude mortality that may exceed 50%.1 2 3 4 Aspiration of bacteria from the upper digestive tract is important in the pathogenesis of this infection.4 5 Two different interventions aimed at decreasing the oral bacterial load are selective decontamination of the digestive tract, involving administration of non-absorbable antibiotics by mouth and through a nasogastric tube, and oral decontamination, which is limited to topical oral application of antibiotics or antiseptics.
Previous meta-analyses of selective decontamination of the digestive tract found a significant reduction in rates of ventilator associated pneumonia among treated patients.6 7 8 9 10 11 12 13 14 The use of this intervention is, however, limited by concern about the emergence of antibiotic resistant bacteria.15 16 17 Oral decontamination alone therefore may be more attractive because it requires only a fraction of the antibiotics used in selective decontamination of the digestive tract. To date, trials of oral decontamination using antibiotics have generated conflicting results, some suggesting benefit18 19w1 and others showing no benefit.w2 w3
One alternative to oral decontamination with antibiotics is to use antiseptics, such as chlorhexidine gluconate or povidone iodine. In contrast to antibiotics, antiseptics act rapidly at multiple target sites and accordingly may be less prone to induce drug resistance.20 Observational studies suggest that antiseptic oral decontamination can reduce ventilator associated pneumonia,21 22 but randomised controlled trials are not convincing.23w4-w6 Recently a meta-analysis of four trials on chlorhexidine failed to show a significant reduction in rates of ventilator associated pneumonia.24 Two subsequent randomised controlled trials, however, suggested benefit from this approach.w7 w8
Current guidelines from the Centers for Disease Control and Prevention recommend topical oral chlorhexidine 0.12% during the perioperative period for adults undergoing cardiac surgery (grade II evidence).3 The routine use of antibiotic or antiseptic oral decontamination for the prevention of ventilator associated pneumonia, however, remains unresolved.3 Despite the lack of firm evidence favouring this preventive intervention, a recent survey across 59 European intensive care units from five countries showed that 61% of the respondents used oral decontamination with chlorhexidine.25
We carried out a systematic review and meta-analysis to estimate the effect of oral decontamination using topical antibiotics or antiseptics on ventilator associated pneumonia and mortality in mechanically ventilated adults.
Methods
With the assistance of a professional librarian we searched for relevant randomised controlled trials using the Ovid version of Medline (1966 to May week 3, 2006) and a maximally sensitive strategy. We modified this search for Embase (1980 to week 21, 2006) and CINAHL (1982 to May week 3, 2006). We also searched CENTRAL (the Cochrane Central Register of Controlled Trials, the Cochrane Library, issue 1, 2006) and the Cochrane Database of Systematic Reviews, issue 1, 2006. We screened previous meta-analyses and the references lists from all retrieved articles for additional studies. Further searches were carried out in two trials registers (www.clinicaltrials.gov/ and www.controlled-trials.com/) and on the web postings from conference proceedings, abstracts, and poster presentations. We also contacted authors and experts in the specialty.
Study selection and data extraction
We included published and unpublished randomised controlled trials testing the effect of oral decontamination on the incidence of pneumonia and mortality in adults requiring mechanical ventilation in an intensive care unit. We considered any type or combination of antibiotics or antiseptics. We had no language restrictions. Trials on selective decontamination of the digestive tract, observational studies, editorials, and commentaries were excluded.
Two independent reviewers (EC and AR) screened all titles and abstracts for inclusion. One reviewer (AR) was blinded to author, journal, institutional affiliation, and date of publication. We then independently assessed each selected reference for detailed evaluation. Interobserver agreement on the selection of articles for inclusion was measured with Cohen's (unweighted) κ statistic.26 Two reviewers (EC and AR) also independently abstracted relevant trial characteristics, and disagreements were resolved by discussion. We contacted authors of the primary studies for clarifications as necessary.
Quality assessment
Two reviewers (EC and AR) independently appraised the quality of included trials. We evaluated randomisation, allocation concealment, blinding techniques, clarity of inclusion and exclusion criteria and outcome definitions, similarity of baseline characteristics, and completeness of follow-up. We considered randomisation to be true if the allocation sequence was generated using computer programs, random number tables, or random drawing of opaque envelopes. Alternate treatment allocation was classified as non-random. Allocation was considered concealed if it involved a telephone call to a central site, used opaque sealed envelopes, or was executed centrally by the pharmacy. Allocation was categorised as unconcealed when described as open or directly managed by the study investigators or when the methods were unclear. A study was considered blinded when patients, caregivers, and data collectors or outcome assessors were blinded, or when it was reported as double blind by the authors. We contacted authors to clarify methodology as necessary.
Data synthesis
We grouped trials according to the specified prophylactic agent used for oral decontamination. The two broad categories were randomised controlled trials in which oral antibiotics were tested against no prophylaxis and oral antiseptics were tested against no prophylaxis.
The primary outcomes were incidence of ventilator associated pneumonia and mortality. We used the authors' definition for ventilator associated pneumonia if it included clinical and radiological criteria. As such, we excluded trials that used the clinical pulmonary infection score alone. We considered mortality in the intensive care unit in the absence of hospital mortality data. Secondary outcomes were the group mean duration of mechanical ventilation and stay in the intensive care unit. We also combined trials on antibiotics and antiseptics for the primary outcomes of ventilator associated pneumonia and mortality, in light of the a priori expectation of a similar magnitude and direction of treatment effect.
Meta-analysis was carried out using Review Manager 4.2 (Cochrane Collaboration, Oxford) and a random effects model.27 The pooled effects estimates for binary variables were expressed as relative risk with 95% confidence interval, whereas continuous variables were expressed as mean differences with 95% confidence intervals. We tested the difference in estimates of treatment effect between the treatment and control groups for each hypothesis using a two sided z test with statistical significance considered at P<0.05. We calculated the number of patients needed to treat (NNT, with 95% confidence interval) to prevent one episode of ventilator associated pneumonia during the period of mechanical ventilation, using the formula:
NNT=1/(RRR×median CER)
where RRR is the summary relative risk reduction and median CER is the median of the control events rates for all trials.
We used Cochran Q and I2 statistics to assess for heterogeneity of results.28 29 We predefined heterogeneity as low, moderate, and high with I2 of above 25%, 50%, and 75%.29 The a priori hypotheses to explain heterogeneity were method of allocation (smaller treatment effect in concealed compared with unconcealed allocation), blinding technique (smaller treatment effect in blinded compared with unblinded studies), patient population (smaller treatment effect in medical or mixed patients compared with selected surgical or trauma patients), and duration of ventilation (smaller treatment effect in patients with mean duration of ventilation of 48 hours or more compared with less than 48 hours). The purpose of the first two analyses was to evaluate whether two critical methodological qualities influenced results.30 We also carried out a post hoc subgroup analysis to investigate the influence of alternative approaches to the diagnosis of ventilator associated pneumonia (quantitative culture of bronchoalveolar lavage fluid or protected specimen brush compared with non-quantitative culture of endotracheal aspirate or other criteria).
We compared relative risk estimates between subgroups using a two sided z test on the log relative risks, and expressed as a ratio of relative risks with its 95% confidence interval.31
The three trials with three arm comparisons were analysed as follows. In two studies,w1 w8 owing to the similarity of the control arms, we pooled them and compared the results with the treatment group. In the third studyw7 we excluded one of the two control arms from analysis because it incorporated both antibiotics and chlorhexidine.
To evaluate potential publication bias we constructed a funnel plot for the primary outcome of ventilator associated pneumonia, using odds ratio as the measure of effect, and visually inspected it for asymmetry. We also carried out Egger's regression intercept and Begg's rank correlation tests to assess this asymmetry formally. Analysis was done using Comprehensive Meta-analysis version 2.2.040 (Biostat, Englewood, NJ). We considered a one tailed P value of less than 0.05 as significant.
Results
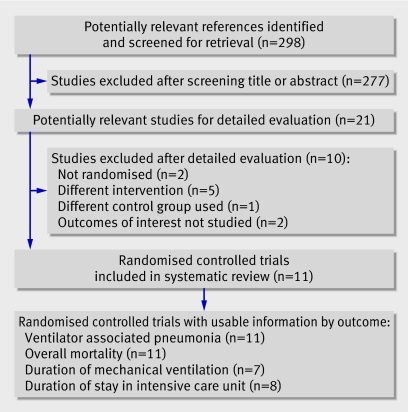
Eleven randomised controlled trials totalling 3242 patients met the inclusion criteria (table 1 and fig 1). Nine were reports published between 1994 and 2006,w1-w9 and two were published in abstract form.w10 w11 Four trials (1098 patients) assessed the effectiveness of antibiotic oral decontamination, whereas seven (2144 patients) evaluated the effectiveness of antiseptic oral decontamination. In the antibiotic category one trial tested Iseganan as the decontaminant.w2 Iseganan is a synthetic variant of a porcine protegrin, which is a natural antibiotic peptide released by neutrophils in response to invasion by microbes. Details of the excluded studies are available on request.18 19 21 23 32 33 34 35 36 37
Table 1.
Characteristics of included trials
| Study | Population | Intervention | Comparison | Outcomes | Follow-up | Funding |
|---|---|---|---|---|---|---|
| Bergmans 2001w1 | Mixed | Orabase with gentamicin, colistin, and vancomycin, 4 times daily until extubation, death, limited to 21 days | Control A, placebo in intensive care unit with patients receiving topical antimicrobial prophylaxis; control B, placebo in intensive care unit with no topical antimicrobial prophylaxis | Ventilator associated pneumonia: clinical, radiological, and bacteriological investigations, including quantitative culture of bronchoalveolar lavage fluid or protected specimen brush. Mortality in hospital | Until extubation or death | Local and industry |
| De Riso 1996w4 | Cardiothoracic (open heart surgery) | Chlorhexidine 0.12% 15 ml preoperatively and twice daily postoperatively until discharge from intensive care or death | Placebo | Ventilator associated pneumonia: Centers for Disease Control and Prevention criteria.‡ Mortality in hospital | Until discharge from intensive care unit or death | Local |
| Fourrier 2000w5* | Medical or surgical | Chlorhexidine 0.2% gel three times daily during stay in intensive care unit until 28 days, discharge from intensive care, or death | Standard treatment | Ventilator associated pneumonia: clinical, radiological, and bacteriological investigations and quantitative culture of tracheal aspirate or bronchoalveolar lavage fluid, or both. Mortality in intensive care unit | Until discharge from intensive care unit or death | Local |
| Fourrier 2005w6*† | 60% medical, 40% surgical | Chlorhexidine 0.2% gel three times daily during stay in intensive care unit until 28 days | Placebo | Ventilator associated pneumonia: clinical, radiological, and bacteriological investigations and quantitative culture of tracheal aspirate or bronchoalveolar lavage fluid, or both. Mortality in intensive care unit by day 28 | Until 28 days in intensive care, discharge from intensive care unit, or death | Local, and industry provided study drug |
| Koeman 2006w7* | Mixed | Treatment A, chlorhexidine 2% in white petroleum vehicle four times daily until diagnosis of ventilator associated pneumonia, death, or extubation; treatment B, chlorhexidine 2% and colistin four times daily | Placebo | Ventilator associated pneumonia: clinical, radiological, and bacteriological investigations and semiquantitative culture of tracheal aspirates. Independent adjudication committee determined if patients had ventilator associated pneumonia. Mortality in intensive care unit | Until extubation, discharge from intensive care unit, or death | Local |
| Kollef 2006w2† | 83% non-trauma, 27% trauma | Iseganan 3 ml (9 mg) six times daily until 14 days. Treatment discontinued if patient developed ventilator associated pneumonia or was extubated | Placebo | Ventilator associated pneumonia: clinical, radiological, and bacteriological investigations, including quantitative culture of bronchoalveolar lavage fluid or non-directed bronchoalveolar lavage fluid. Mortality in intensive care unit by day 14 | Until 21 days or death | Industry |
| Laggner 1994w3 | General intensive care | Gentamicin gel four times daily until extubation. All received oral amphotericin B and oral disinfection with phenylhydragyrum boricum and hexetidine | Placebo | Ventilator associated pneumonia: clinical and radiological investigations and positive culture of tracheal secretions. Mortality in intensive care unit | Until extubation | Not reported |
| MacNaughton 2004w11* | Medical or surgical | Chlorhexidine 0.2% oral rinse twice daily until extubation or death | Placebo | Ventilator associated pneumonia: leucocytosis and pyrexia >38°C; deterioration in arterial blood gases; chest signs; new consolidation on chest radiography; and significant semiquantitative culture of non-directed bronchoalveolar lavage fluid. Definite pneumonia 4/4 if met all four criteria. Mortality in intensive care unit | Not available | Local |
| Rios 2005w10* | Medical or surgical (including trauma) | Polymyxin B and gentamicin gel three times daily until 24 hours after extubation | Placebo | Ventilator associated pneumonia: clinical, radiological, and bacteriological, including positive quantitative culture of tracheal secretions. Mortality in intensive care unit | Until 28 days after ventilator associated pneumonia diagnosis or discharge from intensive care unit, or hospital discharge | Local |
| Segers 2005w9* | Cardiothoracic | Chlorhexidine 0.12%, nasal ointment, and 10 ml oropharynx rinse four times daily on allocation and admission to hospital until extubation or removal of nasogastric tube | Placebo | Ventilator associated pneumonia: Centers for Disease Control and Prevention criteria (no microbiological confirmation required). Mortality in hospital | Until 48 hours after discharge | Local |
| Seguin 2006w8* | Surgical (severe closed head trauma) | Povidone iodine 10% 20 ml reconstituted to 60 ml with sterile water to nasopharynx and oropharynx six times daily until extubation | Control A, saline rinse 60 ml; control B, standard treatment | Ventilator associated pneumonia: clinical, radiological, and bacteriological investigations including positive quantitative culture of bronchoalveolar lavage fluid or non-directed bronchoalveolar lavage fluid. Mortality in intensive care unit | Until discharge from intensive care unit | Not funded |
*Published and unpublished data.
†Trial stopped early.
‡Unclear if clinically defined ventilator associated pneumonia or microbiology confirmed ventilator associated pneumonia.
Fig 1 Flow of studies through trial
All included studies were parallel design randomised controlled trials and were published in English. Most included general mixed patients in intensive care. Nine studies compared active treatment with placebo and twow5 w8 used “standard oral care” as the control. In all trials except five,w1 w2 w4-w6 the prophylactic regimen was given until extubation. Few studies reported on confounding strategies to prevent ventilator associated pneumonia.38 Three trials mentioned semirecumbent positioningw1 w7 w8 and only one trial controlled for route of intubation and management of humidification using a ventilator circuit.w8
The diagnostic criteria for ventilator associated pneumonia differed across trials (table 1). Several trials used quantitative microbiology to confirm ventilator associated pneumonia: threew1 w2 w8 required a quantitative culture of bronchoalveolar lavage fluid or protected specimen brush, two used quantitative cultures of bronchoalveolar lavage fluid or endotracheal aspirate,w5 w6 and one used quantitative cultures of tracheal aspirates.w10 The other trials used either semiquantitative techniquesw3 w7 w11 or did not require microbiological confirmation,w9 whereas in one trial the criteria were unclear.w4 Except for three trials, the inclusion criteria included an anticipated duration of mechanical ventilation of 48 hours or more. Patients were ventilated for a mean duration of more than 48 hours in all but one trial.w9 Seven trials reported duration of mechanical ventilation as means and standard deviations; eight trials reported duration of stay in the intensive care unit as such. One trialw1 reported both of these outcomes as median and range values; these results were not included in the pooled analyses.
Interobserver agreement on the selection of trials for potential inclusion based on reading the titles and abstracts was excellent (Cohen's unweighted κ=0.84, 95% confidence interval 0.64 to 1.03). Interobserver agreement on the inclusion of relevant studies after detailed evaluation was also excellent (κ=1).
Eight of nine authors responded to our requests and provided additional information on trial design, key quality features, and outcome data. Table 2 shows the methodological quality of included trials.
Table 2.
Methodological quality of included trials
| Study | Randomisation | Allocation concealment | Blinding | Explicit inclusion and exclusion criteria | Baseline similarities‡ | % Patients analysed for ventilator associated pneumonia divided by total No of patients randomised | Exclusions after randomisation |
|---|---|---|---|---|---|---|---|
| Bergmansw1 | Unclear | Executed by pharmacy | Described as double blind | Yes | Yes | 92.2 | Early extubation or death (<48 hours) |
| De Risow4 | Computer generated list | Executed by pharmacy | Patients, caregivers, outcome assessors | Yes | Yes | Presumably 100 | Not available |
| Fourrierw5* | Computer generated list, randomisation in block of 4 | Unclear | Described as single blind | Yes | Yes | Presumably 100 | Not available |
| Fourrierw6* | Block randomisation stratified by site | Sealed envelopes by pharmacy | Described as double blind | Yes | Yes | 99.6 | Protocol violation: oral topical antibiotherapy needed |
| Koemanw7* | Computer randomised tables stratified by centre | Executed by pharmacy | Patients, caregivers, data collectors, outcome assessors | Yes | Yes | 100 | None |
| Kollefw2 | Computer generated list | Central telephone | Patients, caregivers, data collectors, outcome assessors | Yes | Yes | 97.8 (only 87.7 completed the study. Unclear if those withdrawn, missing, or lost to follow-up were evaluated for ventilator associated pneumonia) | Did not receive study drug |
| Laggnerw3 | Computer generated randomisation in time blocks† | Open | Described as double blind | Yes | Yes | 76.1 | Early extubation (<5 days), enteral nutrition |
| Macnaughtonw11* | Block randomisation by random table | Executed by pharmacy | Described as double blind (patients, caregivers, investigators) | Yes | Unclear | 100 | None |
| Riosw10* | Random opening of opaque envelopes | Executed by pharmacy | Patients, caregivers, data collectors, outcome assessors | Yes | Yes | 82.8 | Decision to limit therapeutic efforts, death, or early extubation |
| Segersw9* | Computer randomised list | Executed by pharmacy | Patients, caregivers, data collectors, outcome assessors | Yes | Yes | 96.3 | Selective decontamination of digestive tract, withdrew consent, surgery cancelled or death before surgery |
| Seguinw8* | Computer randomised list | Sealed envelopes | Data collectors, outcome assessors | Yes | Yes | 89.1 | Brain death, early extubation |
*Published and unpublished data.
†Information obtained from Liberati et al.14
‡Age, sex, severity of disease, and, where available, systemic antibiotic treatment and ulcer prophylaxis usage.
Primary outcomes
Ventilator associated pneumonia
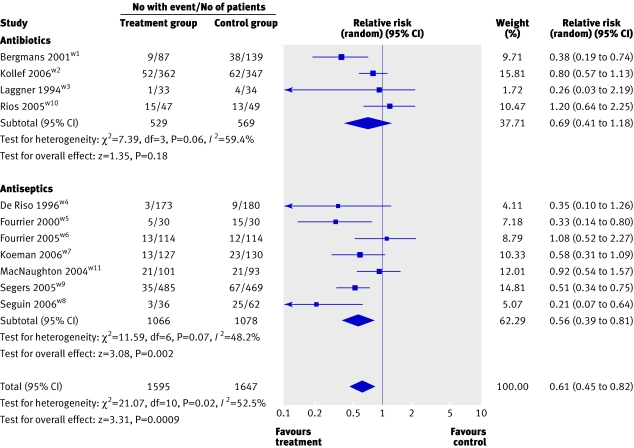
Results from 11 trials (3242 patients) were available to examine the effects of oral decontamination on rates of ventilator associated pneumonia. Meta-analysis of four trials (1098 patients) testing antibiotic oral decontamination did not show a statistically significant reduction in ventilator associated pneumonia rates (relative risk 0.69, 0.41 to 1.18; P=0.18; I2=59.4%; fig 2). Pooled analysis of the seven trials (2144 patients) that tested the effect of antiseptic oral decontamination on ventilator associated pneumonia showed a significant reduction (relative risk 0.56, 0.39 to 0.81; P=0.002; I2=48.2%). The 11 trials combined favoured oral decontamination (relative risk 0.61, 0.45 to 0.82; P<0.001; I2=52.5%). Fourteen patients (NNT 14, 10 to 31) would need to receive oral decontamination with one of these methods to prevent one case of ventilator associated pneumonia.
Fig 2 Forest plot showing effect of oral decontamination prophylaxis compared with no prophylaxis on risk of ventilator associated pneumonia
Table 3 summarises the four a priori subgroup analyses. An informative comparison was possible for only two subgroups in the antiseptic trials, because either none or one comparison group existed for the other subgroups. Blinded trials yielded a more modest treatment effect than unblinded trials; medical or mixed populations also seemed to derive a more modest treatment effect compared with surgical or trauma patients. Table 3 also shows the post hoc subgroup analyses on diagnostic criteria for ventilator associated pneumonia where it was possible to compare the subgroups only in the antibiotics trials. Trials that used quantitative culture of bronchoalveolar lavage fluid observed a trend towards greater treatment effects compared with those that relied on less invasive diagnostic methods.
Table 3.
Subgroup analyses comparing effect of oral decontamination using antibiotic or antiseptic with no prophylaxis on incidence of ventilator associated pneumonia
| Measurement | Antibiotic oral decontamination | Antiseptic oral decontamination | |||||
|---|---|---|---|---|---|---|---|
| Relative risk (95% CI) | No of studies (No of patients) | Ratio of relative risks (95% CI), P value* | Relative risk (95% CI) | No of studies (No of patients) | Ratio of relative risks (95% CI); P value* | ||
| Allocation: | |||||||
| Concealed† | 0.73 (0.42 to 1.28) | 3 (1031) | — | 0.60 (0.40 to 0.89) | 6 (2084) | — | |
| Unconcealed | 0.26 (0.03 to 2.19) | 1 (67) | 0.33 (0.14 to 0.80) | 1 (60) | |||
| Blinding: | |||||||
| Blinded‡ | — | — | NA§ | 0.66 (0.47 to 0.93) | 5 (1986) | 2.36 (1.09 to 5.10); 0.03 | |
| Unblinded | — | — | 0.28 (0.14 to 0.56) | 2 (158) | |||
| Patient population: | |||||||
| Medical or mixed | — | — | NA¶ | 0.70 (0.44 to 1.10) | 4 (739) | 1.67 (0.86 to 3.22); 0.13 | |
| Selected surgical or trauma | — | — | 0.42 (0.26 to 0.67) | 3 (1405) | |||
| Duration of ventilation (hours): | |||||||
| ≥48 | — | — | NA** | 0.56 (0.34 to 0.91) | 6 (1190) | — | |
| <48 | — | — | 0.51 (0.34 to 0.75) | 1 (954) | |||
| Ventilator associated pneumonia diagnostic criteria: | — | ||||||
| Quantitative culture of bronchoalveolar lavage fluid | 0.58 (0.28 to 1.22) | 2 (935) | 0.74 (0.16 to 3.53); P=0.71 | 0.21 (0.07 to 0.64) | 1 (98) | — | |
| Non-quantitative culture of aspirate or others | 0.78 (0.20 to 3.12) | 2 (163) | 0.61 (0.44 to 0.86) | 6 (2046) | |||
*Comparison of estimates in each subgroup (for example, concealed versus unconcealed trials).
†Concealed = reported as open, or unclear.
‡Patients, caregivers, and data collectors or outcome assessors blinded, or reported as double blind.
§None were unblinded.
¶None were surgical or trauma patients.
**None were ventilated for <48 hours.
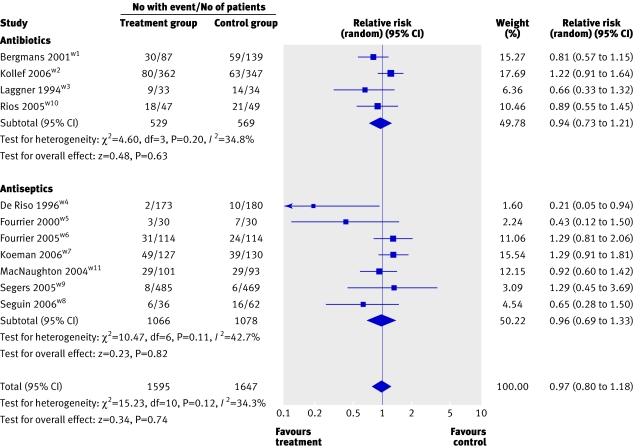
Overall mortality
Results of all 11 trials were available for the analysis of mortality (fig 3). Meta-analysis of the four trials that tested antibiotic prophylaxis found no effect on overall mortality (relative risk 0.94, 0.73 to 1.21; P=0.63; I2=34.8%). The pooled analysis of the seven antiseptic trials (2144 patients) also showed no effect on mortality (0.96, 0.69 to 1.33; P=0.82; I2=42.7%). Pooling the 11 studies produced similar results (0.97, 0.80 to 1.18; P=0.74; I2=34.3%).
Fig 3 Forest plot showing effect of oral decontamination prophylaxis compared with no prophylaxis on overall mortality
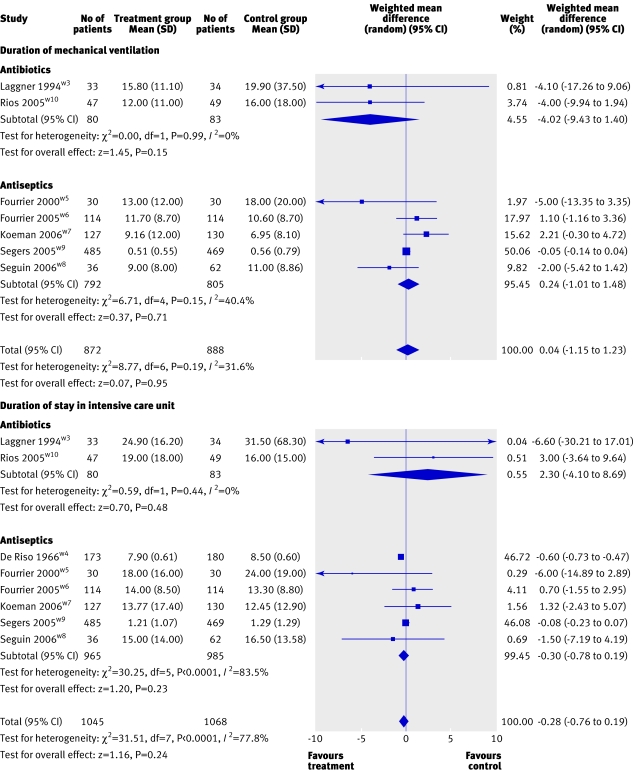
Duration of mechanical ventilation
Overall seven trials (1760 patients) contributed to the analysis of duration of mechanical ventilation. Neither the pooled mean difference for prophylaxis using antibiotics (−4.02 days, −9.43 to 1.40; P=0.15; I2=0%) or antiseptics (0.24 days, −1.01 to 1.48; P=0.71; I2=40.4%) showed an effect on duration of mechanical ventilation. The combined mean difference for all trials was 0.04 days (−1.15 to 1.23; P=0.95; I2=31.6%; fig 4).
Fig 4 Forest plot showing effect of oral decontamination prophylaxis compared with no prophylaxis on duration (days) of mechanical ventilation and duration of stay (days) in an intensive care unit
Duration of stay in intensive care unit
Overall eight trials (2113 patients) contributed to the analysis of the duration of stay in the intensive care unit, which did not seem to be influenced by prophylaxis using either antibiotics (2.30 days,−4.10 to 8.69; P=0.48; I2=0%) or antiseptics (−0.30 days, −0.78 to 0.19; P=0.23; I2=83.5%). The combined mean difference for all trials was −0.28 days (−0.76 to 0.19; P=0.24; I2=77.8%; fig 4).
Publication bias
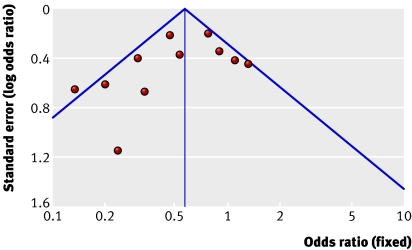
The funnel plot for ventilator associated pneumonia was asymmetrical, suggesting the existence of unpublished small studies with negative findings (fig 5). Formal statistical tests did not, however, support the presence of publication bias: Egger's regression intercept (intercept −1.32, −3.59 to 0.95; one tailed P=0.111) and Begg's rank correlation (Kendall's τ with continuity correction −0.22; one tailed P=0.175).
Fig 5 Funnel plots assessing publication bias for ventilator associated pneumonia
Discussion
The effectiveness of prophylactic oral decontamination to prevent pneumonia in patients undergoing mechanical ventilation has remained controversial since its introduction, due partly to discordant results of individual trials. We analysed antibiotic and antiseptic prophylaxis as two distinct approaches to oral decontamination. Our results suggest that antiseptic oral decontamination is effective at preventing ventilator associated pneumonia. More evidence is needed before firm conclusions can be made about antibiotic oral decontamination, although effects may be similar. This review included twice as many participants in the antiseptic trials than antibiotic trials, reflecting more precise results for the analysis of antiseptics.
We found that neither antibiotic nor antiseptic oral decontamination influenced overall mortality, duration of mechanical ventilation, or duration of stay in an intensive care unit. Our review was underpowered to detect any effect on mortality, and the small sample size limited the interpretation of the secondary outcomes.
Comparison with previous studies
Previous meta-analyses examining the effect of prophylaxis using selective decontamination of the digestive tract reported a significant reduction in the incidence of ventilator associated pneumonia.6 7 8 9 10 11 12 13 14 The most recent meta-analysis indicated that such an intervention combined with prophylactic intravenous antibiotics reduces overall mortality.14 In comparison our review suggests that oral antiseptic prophylaxis alone can significantly reduce the incidence of ventilator associated pneumonia, but not mortality. Our meta-analysis on antiseptics differs from the findings of Pineda et al, who pooled four trials on chlorhexidine and did not report lower rates of ventilator associated pneumonia (odds ratio 0.42, 0.16-1.06; P=0.07).24 Our results also extend those of Chlebicki et al, who did not find a statistically significant benefit using the more conservative random effects model after pooling seven trials on chlorhexidine (relative risk 0.70, 0.47-1.04; P=0.07), although their results were significant with the fixed effects model.39 Our systematic review included a larger dataset with two more recent trials,w8 w9 involved clarification of data from several authors, and explored heterogeneity with more subgroup analyses.
Possible explanations and implications
The lack of effect on secondary outcomes may raise concern about the accuracy with which ventilator associated pneumonia was diagnosed, given that the antiseptic trials, despite showing a substantial reduction in ventilator associated pneumonia rates, failed to show similar benefit for these secondary outcomes. It is possible that the combination of clinical, radiological, and microbiological criteria without the use of quantitative investigations using cultures of bronchoalveolar lavage fluid, which may have a high sensitivity but low specificity,40 may contribute to an overestimation of the ventilator associated pneumonia rates in these trials, and a greater observed treatment effect.
To ensure that the lack of effect on patients' secondary outcomes did not arise from the differences in the diagnostic criteria used by the primary trials, we carried out a post hoc subgroup analysis on the basis of diagnostic criteria for ventilator associated pneumonia (differentiating between trials using invasive quantitative culture of bronchoalveolar lavage fluid or protected specimen brush versus other less invasive approaches). Only one of the antiseptic trials used invasive quantitative criteria, rendering further analysis not possible. Our analysis for the antibiotic trials was inconclusive, showing a trend towards a greater treatment effect for the trials that used the more invasive diagnostic criteria (table 3). An analysis combining all trials on antibiotics and antiseptics also suggested the same trend (invasive quantitative criteria's relative risk 0.45, 0.21 to 0.98 v less invasive criteria's relative risk 0.66, 0.47 to 0.93), although the comparison of these relative risks was not conclusive (ratio of relative risks 0.68, 0.29 to 1.58; P=0.37). Nevertheless, a recent large multicentre trial found no difference in clinical outcomes or subsequent overall antibiotic use when a diagnostic approach of quantitative culture of bronchoalveolar lavage fluid was compared with non-quantitative culture of endotracheal aspirate among non-immunocompromised patients not suspected of harbouring high risk organisms.41
Our a priori subgroup analyses suggest that trials with an unblinded design and those enrolling surgical or trauma patients tended to yield qualitatively larger treatment effects than blinded trials and those enrolling medical or mixed critically ill patients. The former result is consistent with previous work showing that trials of lower methodological quality tend to report greater treatment effects.42 Specific surgical or trauma patients often have fewer comorbidities than medical or mixed patients, which may explain the trend towards a greater treatment effect in the former population. However, these subgroup results are best viewed as hypothesis generating.
The finding that antiseptic oral decontamination can reduce the incidence of ventilator associated pneumonia could have important implications for lower healthcare costs and a reduced risk of antibiotic resistance compared with the use of antibiotics. It may not be prudent to adopt this practice routinely for all critically ill patients until strong data on the long term risk of selecting antiseptic and antibiotic resistant organisms are available. Nevertheless, antiseptic oral decontamination seems promising.
Strengths and weaknesses of the study
The strengths of this review include the comprehensive search for relevant randomised controlled trials, duplicate screening, selection, assessment of methodological quality and data abstraction, and use of the random effects model (which takes heterogeneity into account) to combine trial results. We separated and then combined the antibiotic and antiseptic trials, anticipating that the underlying pathophysiology could lead to a similar treatment effect across the trials,43 and because an overall treatment effect is of interest in examining the relation between oral flora and lung infection during critical illness.
We inspected funnel plots to evaluate potential publication bias for ventilator associated pneumonia. We also undertook formal statistical tests. These did not show the presence of publication bias for the combined 11 antibiotics and antiseptic trials. However, the power of these tests is generally low. Although our literature search was comprehensive, it is possible that we missed other relevant trials. In addition, these trials were heterogeneous with respect to populations enrolled, regimens used, outcome definitions, and analysis strategies, contributing to differing relative risks across the trials. Other limitations of the trials we included were exclusions after randomisation, mainly due to early extubation, early deaths, or protocol violations. Some trials did not explicitly report whether the number of patients analysed reflected the total number of patients randomised (table 2) such that we were unable to abstract the intention to treat analyses from all trials. Finally, we could not obtain unpublished data from some authors on the mean duration of mechanical ventilation and stay in an intensive care unit.
Unanswered questions and future research
Our systematic review supports the use of antiseptic oral decontamination. Research to date does not address which antiseptic is preferred, since all but one trial evaluated chlorhexidine. We cannot recommend precise methods for chlorhexidine administration owing to the wide variation of treatment regimens among studies. These included varying concentrations (0.12%, 0.2%, 2%), sites of application, forms of agent (oral rinse, gel), and frequencies and techniques of application. Nevertheless, our findings suggest that the concentration of chlorhexidine may be a consideration. In trials with cardiac surgery patients at low risk for developing ventilator associated pneumonia owing to a short duration of intubation, chlorhexidine 0.12% was effective in reducing ventilator associated pneumonia.w4 w9 However, among medical or mixed intensive care populations, a higher concentration may be necessary. Chlorhexidine was not effective in most of these trials at 0.2% concentrationw6 w11 but was effective at 2%.w7 As for the only trial that used povidone iodine, the agent was found to be effective in preventing ventilator associated pneumonia among 98 patients with head injuries with a persistent score of 8 or less on the Glasgow coma scale requiring mechanical ventilation for 48 hours or more.w8
To our knowledge no trial directly compares antiseptic with antibiotic oral decontamination. Further investigations comparing antibiotic with antiseptic oral decontamination while incorporating stringent infection surveillance would be worthwhile. Whether either antibiotic or antiseptic oral decontamination favourably influence important patient outcomes such as duration of mechanical ventilation or duration of stay in the intensive care unit should be evaluated in rigorously designed and adequately powered randomised trials.
Conclusions
This systematic review suggests that in mechanically ventilated patients, antiseptic oral decontamination prophylaxis reduces the incidence of ventilator associated pneumonia. More evidence is needed before firm conclusions can be made on the effect of antibiotic oral decontamination. These results should be interpreted in light of the moderate heterogeneity of trial results and possible publication bias. Neither of these two approaches to decontamination seems to affect mortality, duration of mechanical ventilation, or stay in the intensive care unit, although these trials are underpowered for these latter outcomes, and the summary of trials to date does not yet represent the optimum information size.44 Therefore more evidence is needed before firm conclusions can be made on the full effect of oral decontamination using antiseptics and, particularly, antibiotics.
What is already known on this topic
Selective decontamination of the digestive tract reduces the incidence of ventilator associated pneumonia
Oral decontamination requires only a fraction of the antibiotics used for selective decontamination
What this study adds
Oral decontamination using antiseptics reduces the incidence of ventilator associated pneumonia
Neither antibiotic nor antiseptic oral decontamination reduces overall mortality or duration of mechanical ventilation or stay in intensive care
Supplementary Material
We thank Patrique Segers, Mirelle Koeman, Carlos Apezteguia, Fernando Rios, Peter Macnaughton, Francois Fourrier, Philippe Seguin, and José Manuel Rodriguez-Roldan for providing additional information on their trials.
Contributors: All authors contributed to the concept, design, and critical revision of the manuscript. EC and AR carried out the search, selected the articles, and extracted the data. EC carried out the statistical analyses with input from AR, drafted the review, and is guarantor.
Funding: EC is supported by a scholarship award from Tan Tock Seng Hospital and National Healthcare Group HMDP, Singapore. AR is supported by a postdoctoral fellowship award from the Fonds de Recherche en Santé du Québec and from Université Laval. DJC holds a Canada Research Chair. Statement of the independence of researchers from funders: The authors' work was independent of the funders (the funding source has no involvement).
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867-903. [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002;122:2115-21. [DOI] [PubMed] [Google Scholar]
- 3.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1-36. [PubMed] [Google Scholar]
- 4.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [DOI] [PubMed] [Google Scholar]
- 5.Estes RJ, Meduri GU. The pathogenesis of ventilator-associated pneumonia: I. Mechanisms of bacterial transcolonisation and airway inoculation. Intensive Care Med 1995;21:365-83. [DOI] [PubMed] [Google Scholar]
- 6.Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med 1984;10:185-92. [DOI] [PubMed] [Google Scholar]
- 7.Vandenbroucke-Grauls CM, Vandenbroucke JP. Effect of selective decontamination of the digestive tract on respiratory tract infections and mortality in the intensive care unit. Lancet 1991;338:859-62. [DOI] [PubMed] [Google Scholar]
- 8.Selective Decontamination of the Digestive Tract Trialists' Collaborative Group. Meta-analysis of randomised controlled trials of selective decontamination of the digestive tract. BMJ 1993;307:525-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heyland DK, Cook DJ, Jaeschke R, Griffith L, Lee HN, Guyatt GH. Selective decontamination of the digestive tract. An overview. Chest 1994;105:1221-9. [DOI] [PubMed] [Google Scholar]
- 10.Kollef MH. The role of selective digestive tract decontamination on mortality and respiratory tract infections. A meta-analysis. Chest 1994;105:1101-8. [DOI] [PubMed] [Google Scholar]
- 11.Hurley JC. Prophylaxis with enteral antibiotics in ventilated patients: selective decontamination or selective cross-infection? Antimicrob Agents Chemother 1995;39:941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amico R, Pifferi S, Leonetti C, Torri V, Tinazzi A, Liberati A. Effectiveness of antibiotic prophylaxis in critically ill adult patients: systematic review of randomised controlled trials. BMJ 1998;316:1275-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathens AB, Marshall JC. Selective decontamination of the digestive tract in surgical patients: A systematic review of the evidence. Arch Surg 1999;134:170-6. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, D'Amico R, Pifferi, Torri V, Brazzi L. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 2004;(1):CD000022. [DOI] [PubMed]
- 15.Kollef MH. Opinion: the clinical use of selective digestive decontamination. Crit Care 2000;4:327-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gastinne H, Wolff M, Delatour F, Faurisson F, Chevret S. A controlled trial in intensive care units of selective decontamination of the digestive tract with nonabsorbable antibiotics. The French Study Group on Selective Decontamination of the Digestive Tract. N Engl J Med 1992;326:594-9. [DOI] [PubMed] [Google Scholar]
- 17.Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, et al. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann Intern Med 1989;110:873-81. [DOI] [PubMed] [Google Scholar]
- 18.Abele-Horn M, Dauber A, Bauernfeind A, Russwurm W, Seyfarth-Metzger I, Gleich P, et al. Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination (SOD). Intensive Care Med 1997;23:187-95. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Roldan JM, Altuna-Cuesta A, Lopez A, Carrillo A, Garcia J, Leon J, et al. Prevention of nosocomial lung infection in ventilated patients: use of an antimicrobial pharyngeal nonabsorbable paste. Crit Care Med 1990;18:1239-42. [DOI] [PubMed] [Google Scholar]
- 20.Pittet D. Improving compliance with hand hygiene. In: Wenzel RP, ed. Prevention and control of nosocomial infections, 4th ed. Philadelphia: Lippincott Williams, and Wilkins, 2003:532-3.
- 21.Genuit T, Bochicchio G, Napolitano LM, McCarter RJ, Roghman MC. Prophylactic chlorhexidine oral rinse decreases ventilator-associated pneumonia in surgical ICU patients. Surg Infect (Larchmt) 2001;2:5-18. [DOI] [PubMed] [Google Scholar]
- 22.Mori H, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med 2006;32:230-6. [DOI] [PubMed] [Google Scholar]
- 23.Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care 2002;11:567-70. [PubMed] [Google Scholar]
- 24.Pineda LA, Saliba RG, El Solh AA. Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a meta-analysis. Crit Care 2006;10:R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rello J, Koulenti D, Blot S, Sierra R, Diaz E, De Waale J, et al. Oral care practices in intensive care units. A survey of 59 European intensive care units. Intensive Care Med (In press). [DOI] [PubMed]
- 26.Fleiss J.L., Cohen J. The equivalence of weighed kappa and the intraclass correlation coefficient as measures of realibility. Educ Psychol Meas 1973;33:613-9. [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
- 31.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camus C, Bellissant E, Sebille V, Perrotin D, Garo B, Legras A, et al. Prevention of acquired infections in intubated patients with the combination of two decontamination regimens. Crit Care Med 2005;33:307-14. [DOI] [PubMed] [Google Scholar]
- 33.Grap MJ, Munro CL, Elswick RK Jr, Sessler CN, Ward KR. Duration of action of a single, early oral application of chlorhexidine on oral microbial flora in mechanically ventilated patients: a pilot study. Heart Lung 2004;33:83-91. [DOI] [PubMed] [Google Scholar]
- 34.Kerver AJ, Rommes JH, Mevissen-Verhage EA, Hulstaert PF, Vos A, Verhoef J, et al. Prevention of colonization and infection in critically ill patients: a prospective randomised study. Crit Care Med 1988;16:1087-93. [DOI] [PubMed] [Google Scholar]
- 35.Ogata J, Minami K, Miyamoto H, Horishita T, Ogawa M, Sata T, et al. Gargling with povidone-iodine reduces the transport of bacteria during oral intubation. Can J Anaesth 2004;51:932-6. [DOI] [PubMed] [Google Scholar]
- 36.Pugin J, Auckenthaler R, Lew DP, Suter PM. Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia. A randomised, placebo-controlled, double-blind clinical trial. JAMA 1991;265:2704-10. [PubMed] [Google Scholar]
- 37.Silvestri L, van Saene HK, Milanese M, Fontana F, Gregori D, Oblach L, et al. Prevention of MRSA pneumonia by oral vancomycin decontamination: a randomised trial. Eur Respir J 2004;23:921-6. [DOI] [PubMed] [Google Scholar]
- 38.Dodek P, Keenan S, Cook D, Heyland D, Jacka M, Hand L, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med 2004;141:305-13. [DOI] [PubMed] [Google Scholar]
- 39.Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med 2007;35:595-602. [DOI] [PubMed] [Google Scholar]
- 40.Pingleton SK, Fagon JY, Leeper KV Jr. Patient selection for clinical investigation of ventilator-associated pneumonia. Criteria for evaluating diagnostic techniques. Chest 1992;102(5 Suppl 1):S553-6. [DOI] [PubMed] [Google Scholar]
- 41.Canadian Critical Care Trials Group, Heyland D, Cook DJ, Dodek P, Muscedere J, Day A. A randomised trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med 2006;355:2619-30. [DOI] [PubMed] [Google Scholar]
- 42.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
- 43.Hatala R, Keitz S, Wyer P, Guyatt G, Evidence-Based Medicine Teaching Tips Working Group. Tips for learners of evidence-based medicine: 4. Assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. CMAJ 2005;172:661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devereaux PJ, Beattie WS, Choi PT, Badner NH, Guyatt GH, Villar JC, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005;331:313-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.