The clinical problem
After an ischaemic stroke or transient ischaemic attack (TIA), patients are at high risk of subsequent stroke and other vascular events, such as myocardial infarction. Strategies to prevent vascular events (stroke, myocardial infarction, or vascular death) in such patients include using aspirin, which is the most widely tested single antiplatelet drug for this purpose.1 2 Good evidence now exists, however, that adding dipyridamole to aspirin further reduces the risk in patients who have had an ischaemic stroke or transient ischaemic attack.
KEY POINTS
• In patients with a prior ischaemic stroke or transient ischaemic attack, adding the antiplatelet drug dipyridamole (modified release formulation, 200 mg twice daily) to aspirin reduces the relative risk of vascular events (stroke, myocardial infarction, or vascular death) by a fifth
• In patients already receiving current secondary preventive treatment, the average annual risk of a vascular event is no more than 5%; adding dipyridamole prevents one further vascular event for every 100 patients treated each year
• Headache may occur in up to a third of people taking dipyridamole but usually settles in one to two weeks
The evidence for change
Sources of evidence are shown in the box.1 2 3
Sources of evidence
I identified relevant randomised trials and systematic reviews from the sources shown:
• Antithrombotic Trialists Collaboration database of antiplatelet trials1
• Cochrane Library
• Health Technology Assessment report on clopidogrel and dipyridamole3
• Regular (approximately annual) searches for randomised trials and systematic reviews relevant to interventions used in secondary prevention after stroke or transient ischaemic attack conducted by the BMJ Clinical Evidence team2
• My own collection of publications from scanning a range of general medical and stroke related journals
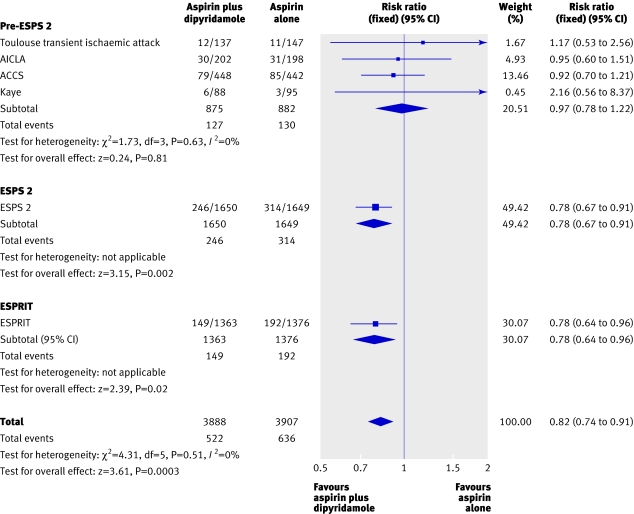
The second European stroke prevention study (ESPS-2) found, in 3299 patients with a prior ischaemic stroke or transient ischaemic attack, that dipyridamole plus aspirin significantly reduced the relative risk of vascular events by about a fifth compared with aspirin alone (table, figure).1 4 5 6 However, pooled data from previous randomised trials in several thousand patients at high risk of vascular events (about 1800 of whom had a prior ischaemic stroke or transient ischaemic attack) did not find that adding dipyridamole reduced vascular events (figure).1 5 6 The contrasting ESPS-2 results were attributed to chance; the very low daily dose (50 mg) of aspirin used compared with previous trials; or the particular daily dose and preparation (400 mg modified release v ≤300 mg standard preparation) of dipyridamole used.1 7
Pivotal randomised trials of aspirin versus aspirin plus dipyridamole
| Second European stroke prevention study (ESPS-2) | European/Australian stroke prevention in reversible ischaemia trial (ESPRIT) | |
|---|---|---|
| Primary publication | 1996 | 2006 |
| Type of trial | Randomised, placebo controlled | Randomised, open control |
| Participants | Adult patients within 3 months of an ischaemic stroke or transient ischaemic attack | Adult patients 0-6 months after minor ischaemic stroke or transient ischaemic attack of presumed arterial origin (patients with a cardioembolic source such as atrial fibrillation excluded) |
| Mean age (years) | 67 | 63 |
| Sex (percentage male) | 58 | 65 |
| Randomisation | 3299 patients randomised between relevant treatment arms: aspirin 25 mg twice daily; aspirin 25 mg twice daily plus modified release dipyridamole 200 mg twice daily | 2739 patients randomised between relevant treatment arms: aspirin 30-325 mg (median 75 mg) daily; aspirin 30-325 mg daily plus dipyridamole 200 mg twice daily (85% used modified release preparation) |
| Follow-up (years) | 2 | 3.5 |
| Average annual risk of vascular events in aspirin only treatment arm (%) | 10 | 5 |
| Outcome of aspirin plus dipyridamole v aspirin alone | Significantly reduced vascular events (relative risk 0.78, 95% confidence interval 0.67 to 0.91)— as a result of significant reduction in stroke, with no detectable effect on either myocardial infarction or vascular death (although numbers of non-stroke outcomes were small) | Significantly reduced vascular events (0.78, 95% confidence interval 0.64 to 0.96)—as a result of reductions in each of ischaemic stroke, cardiac events, and vascular death, although none of these was independently significant. Benefits continued to accrue during follow-up for up to five years. No significant differences were seen in the relative effects of treatment according to several characteristics, including age, sex , history of ischaemic heart disease, and aspirin dose |
Meta-analysis of randomised trials of aspirin plus dipyridamole versus aspirin alone in patients with a prior ischaemic stroke or transient ischaemic attack (outcome: stroke, myocardial infarction, or vascular death) (adapted from the ESPRIT Study Group5)
The recently published European/Australasian stroke prevention in reversible ischaemia trial (ESPRIT) compared aspirin plus dipyridamole versus aspirin alone in 2739 patients with a prior ischaemic stroke or transient ischaemic attack.5 The resulting relative reduction in vascular events was the same as in ESPS-2 (table, figure), and a meta-analysis of all randomised trials comparing the combination with aspirin alone in patients with a prior ischaemic stroke or transient ischaemic attack largely reflected the results of the ESPS-2 and ESPRIT trials (figure ).5
Assuming a baseline risk of vascular events of 5% a year, the annual risk in the aspirin only arm of ESPRIT (reflecting well the risk in patients already receiving current secondary preventive treatments), the addition of dipyridamole would prevent about 10 vascular events per 1000 patients treated per year).5
Neither large trial found an excess of major bleeding in patients allocated the combination compared with aspirin alone. However, both reported a higher rate of premature cessation of treatment in the combination than in the aspirin arm, mainly as a result of adverse effects (particularly dipyridamole induced headache, which may occur in up to a third of people receiving dipyridamole but usually settles in one to two weeks and might be reduced by dipyridamole dose titration).4 5 8
Barriers to change
Doctors or patients may perceive that the small absolute benefit is not worth while, and adherence may be limited by adverse effects and the difficulties for a predominantly elderly population to take additional medication. As rapid intravenous injection of dipyridamole reduces blood pressure when it is used as a coronary vasodilator in stress echocardiography and thallium imaging, anxieties have been expressed about dipyridamole in patients with ischaemic heart disease.7 However, in ESPRIT, long term oral dipyridamole did not affect blood pressure, and the benefits of adding dipyridamole to aspirin were similar in those with and without ischaemic heart disease.5 9 Cost may be a barrier, as in the United Kingdom modified release dipyridamole 200 mg twice daily costs £102 (€150; $200) a patient per year, compared with £5 a patient per year for aspirin 75 mg daily.10
How should we change our practice?
Ensure first that:
• The diagnosis of ischaemic stroke or transient ischaemic attack is correct (which generally requires prompt specialist assessment and appropriate investigations);
• Existing secondary preventive strategies (such as lifestyle advice, aspirin, and reduction in cholesterol and blood pressure) have been or are being considered and used where appropriate;
• No reason (such as atrial fibrillation) exists to consider anticoagulants instead of antiplatelet treatment;
• The patient is already taking and tolerating aspirin, the most appropriate dose being 75-150 mg daily as higher doses produce more gastrointestinal side effects and lower doses may be somewhat less effective.1 7
Then discuss with the patient (or proxy) the likely absolute benefit of adding modified release dipyridamole 200 mg twice daily (that is, on average about a 1 in 100 chance per year of benefiting) versus the potential for adverse effects and the inconvenience of extra pills. Start dipyridamole if the patient wishes; it can be continued long term if tolerated and if no contraindications develop and funding allows.
Competing interests: I was involved in the cited report on clopidogrel and dipyridamole3 and in planning a trial (of aspirin alone or with these agents) that did not proceed as a potential sponsor wished to modify it. I am on the Antithrombotic Trialists' Collaboration's steering committee and received £500 from Sanofi in 1998 to speak on the Trialists' results at a general practitioners' educational meeting. I'm also a member of the No Free Lunch movement.
References
- 1.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for the prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lip GYH, Rothwell P, Sudlow C. Stroke prevention. Clinical evidence. www.clinicalevidence.org/ceweb/conditions/cvd/0207/0207.jspwww.clinicalevidence.org/ceweb/conditions/cvd/0207/0207.jsp [PubMed]
- 3.Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al. Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation. Health Technol Assess 2004;8(38):1-196. [DOI] [PubMed] [Google Scholar]
- 4.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European secondary prevention study 2: dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996;143:1-13. [DOI] [PubMed] [Google Scholar]
- 5.ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006;367:1665-73. [DOI] [PubMed] [Google Scholar]
- 6.De Schryver ELLM, Algra A, van Gijn J. Dipyridamole for preventing stroke and other vascular events in patients with vascular disease. Cochrane Database Syst Rev 2006;(2):CD001820. [DOI] [PubMed]
- 7.Sudlow C. What is the role of dipyridamole in long term secondary prevention after an ischaemic stroke or transient ischaemic attack? CMA J 2005;173:1024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindgren A, Husted S, Staaf G, Ziegler B. Dipyridamole and headache: a pilot study of initial dose titration. J Neurol Sci 2004;223:179-84. [DOI] [PubMed] [Google Scholar]
- 9.De Schryver ELLM, for the ESPRIT Study Group. Dipyridamole in stroke prevention. Effect of dipyridamole on blood pressure. Stroke 2003;34:2339-42. [DOI] [PubMed] [Google Scholar]
- 10.British National Formulary. www.bnf.org/bnf/bnf/current/index.htm