Abstract
The adult reproductive axis is driven by an intermittent discharge of gonadotropin-releasing hormone (GnRH) generated by a network of hypothalamic neurons known as the GnRH pulse generator. Although this signal generator is operational in infant primates, puberty in these species is delayed by activation shortly after birth of a central neural mechanism that holds GnRH release in check during juvenile development. Here, we show that, in the male rhesus monkey, the postnatal pattern in GnRH pulse generator activity is inversely related to that in neuropeptide Y (NPY) gene and protein expression in the mediobasal hypothalamus and that central administration of an NPY Y1 receptor antagonist to juvenile animals elicits precocious GnRH release. Cell imaging indicated that the developmentally regulated NPY neurons may be located in regions dorsal to the arcuate nucleus. These findings lead us to propose that NPY is a fundamental component of the neurobiological brake restraining the onset of puberty in primates.
To investigate the neurobiology of the restraint that is imposed on pulsatile gonadotropin-releasing hormone (GnRH) release in prepubertal primates, we first examined changes in hypothalamic gene expression during the juvenile–pubertal transition, the developmental stage when the GnRH pulse generator (1) is being released from check, which, in the rhesus monkey, occurs at approximately 2–3 yr of age (2, 3). Because the gonads do not dictate the temporal pattern of GnRH pulse generator activity during primate development (2), agonadal monkeys were used. This eliminated any secondary molecular changes in the hypothalamus, which, in intact animals, may be anticipated to occur in response to the action of increased gonadal steroid secretion at the time of puberty, and which may therefore confound identification of the genes that underlie removal of the prepubertal brake on GnRH release. The male was used because the prepubertal hiatus in pulsatile GnRH release is more marked than that in the female (2), and it was reasoned that the components of the brake would be correspondingly exaggerated, and therefore perhaps easier to identify for the first time.
Because pubertal changes in GnRH and γ-aminobutyric acid (GABA) contents in hypothalamic perfusates are inversely related in the female monkey (4), and because inhibition of GABA synthesis or action before menarche leads to precocious GnRH release (5), levels of the mRNAs encoding the GABA-synthesizing enzymes glutamic acid decarboxylase (GAD)65 and GAD67 were studied. Similarly, expression of transforming growth factor α (TGFα), a glial-derived growth factor implicated in the control of puberty in rat (6), was examined because TGFα mRNA levels throughout postnatal development in the female macaque parallel GnRH pulse generator activity (7). mRNA levels encoding neuropeptide Y (NPY) were studied because intracerebroventricular (i.c.v.) administration of this peptide to adult female monkeys is inhibitory to pulsatile GnRH release (8). Finally, this experiment provided an opportunity to reevaluate the conclusion that GnRH gene expression is not up-regulated at the time of puberty in monkeys (7, 9).
As the foregoing study unfolded, results were obtained that prompted additional investigation of (i) NPY expression during the neonatal–juvenile transition, when the GnRH pulse generator is brought into check, and (ii) the action of NPY receptor antagonism on GnRH release in the juvenile. Together, the results provide evidence for the view that NPY is a major component of the neurobiological brake that holds the primate GnRH pulse generator in check during juvenile development.
Materials and Methods
Animals.
Thirty-three male rhesus monkeys (Macaca mulatta) were used. The majority of animals (n = 29) were housed with their mothers in individual cages until killed as neonates (n = 4, Exp. 2) or until 4.5–9 mo of age, at which time they were separated from their mothers and maintained either as pairs in individual cages (n = 4, Exp. 2) or as social groups. Monkeys reared in groups were placed in individual cages for a period of at least 6 wk before initiation of Exps. 1, 3, and 4. Four monkeys were purchased at 1 yr or more of age and housed in individual cages. Animals in individual cages were maintained under a controlled photoperiod (lights on, 0700–1900) and ambient temperature of 20°C (3). These animals were fed at approximately 1100 with Purina Monkey Chow and at 1500 with fruit; they were killed between 0900 and 1100, before feeding. Neonatal monkeys were also killed at this time. All animals were maintained in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and protocols were approved by the Institutional Animal Care and Use Committee.
Reagents.
GnRH (luteinizing hormone-releasing hormone GMP-26 code no. 230-110-40) and acyline were synthesized at the Salk Institute (Contract N01-HD-0-2906) and Bioqual (Rockville, MD), respectively. Both were made available by the Contraception and Reproductive Health Branch, Center for Population Research, National Institute of Child Health and Human Development. Human (h) NPY and the NPY Y1 receptor antagonist (1229U91) were obtained from Peninsula Laboratories and Glaxo Wellcome, respectively. Artificial cerebrospinal fluid (aCSF) was prepared by GIBCO to previous specifications (10).
Surgery.
Neonatal and juvenile animals were orchidectomized under ketamine hydrochloride and pentobarbital anesthesia, respectively (3, 11). To withdraw sequential blood samples and to administer GnRH, monkeys were implanted with an internal jugular vein catheter, fitted with a jacket and tether, and housed in remote sampling cages (3). Stainless steel (22-gauge) lateral i.c.v. cannulae were implanted by using the procedure of Larsen et al. (12): after visualization of the ventricular system with radiopaque dye, the needle used to inject dye was removed and replaced with the cannula that was cemented to the cranium. A silicone catheter (0.6 mm i.d. × 2.0 mm o.d.) attached to the i.c.v. cannula was led with the i.v. line through the tether to the swivel device on the cage top.
Blood Sampling.
Blood samples were collected either by femoral venipuncture at approximately biweekly intervals after sedation with ketamine hydrochloride (approximately 10 mg/kg of body weight i.m.) or by venous catheters without sedation.
RIA.
Luteinizing hormone (LH) concentrations were estimated by a double-antibody RIA using recombinant monkey LH (AFP6936A) for standard and radioiodinated trace and a polyclonal rabbit antiserum (AFP342994) raised against the recombinant LH as first antibody. These reagents were provided by the National Hormone and Pituitary Program. The sensitivity of the LH RIA was 0.09 ng/ml, and the mean intra- and interassay coefficient of variation was <6% and <10%, respectively. Hypothalamic NPY content was determined by an RIA using hNPY as standard and iodinated trace, and a polyclonal rabbit antiserum (RA-31) as previously described (13, 14). Hypothalamic GnRH content was estimated by a double-antibody RIA using the decapeptide as standard and iodinated trace, and a polyclonal rabbit antiserum (R1245) previously characterized (15). NPY and GnRH contents were determined in single assays and normalized to protein content measured in the Bradford assay (Bio-Rad).
RNase Protection Assays.
mRNA levels were quantitated by RNase protection assay as previously described (16), using 32P-labeled riboprobes generated from a 204-bp fragment of monkey GnRH and 364-bp fragment of monkey TGFα cDNAs (7); a 382-bp fragment of monkey GAD65 and a 210-bp fragment of monkey GAD67 cDNAs (5); a 511-bp fragment of rat NPY cDNA (17); a 521-bp fragment of hNPY cDNA (18); and a 117-bp fragment of rat cyclophilin (Cyclo) cDNA (19). The integrated OD of the mRNA hybrids was quantified by Molecular Analyst Software (Bio-Rad) after background subtraction, normalized to the Cyclo hybrid, and expressed as relative OD.
In Situ Hybridization.
In situ hybridization on 25-μm hypothalamic sections was conducted after acetylation, washing in 0.2× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) + 0.02% diethyl pyrocarbonate (DEP), and dehydration. After treatment with prehybridization buffer, sections were incubated with 33P-labeled sense or antisense hNPY riboprobe (0.4 pmol per section). Hybridization proceeded for 2 days at 50°C. Sections were rinsed with 0.1× SSC for 1 h at 60°C, and then with 0.1× SSC overnight at 42°C. After hydration, sections were dried, dipped in Kodak NTB-3 emulsion at 44°C, and stored in dry, light-tight boxes at 4°C for 7 days.
Statistics.
Differences in mean LH levels were determined by using a one-way ANOVA. For all other parameters, the nonparametric Mann–Whitney U test was used. Differences were considered significant if P < 0.05.
Exp. 1: mRNA and Peptide Levels During the Juvenile–Pubertal Transition.
Eight prepubertal monkeys (15–19 mo of age) were orchidectomized. Four animals (juvenile group) were killed within 10 wk of castration, when plasma LH concentrations were undetectable and well before reaugmentation of pulsatile GnRH release was to be anticipated. The remaining monkeys (pubertal group) were killed after the pubertal reaugmentation of pulsatile GnRH release had been established by tracking LH levels. Hypothalami were collected and divided by a coronal cut at the caudal boundary of the optic chiasm separating the hypothalamus into a rostral and caudal block containing the anterior hypothalamic/preoptic area (POA) and the mediobasal hypothalamus (MBH), respectively. mRNA and protein were extracted with RNAzol (RNA STAT-60).
Exp. 2: NPY mRNA and Peptide Levels During the Neonatal–Juvenile Transition.
Eight monkeys were castrated at 1 wk of age; four were killed 4–7 wk later when GnRH pulse generator activity was robust, and four were killed at 12–15 mo of age after verifying that the prepubertal restraint on pulsatile GnRH had been applied. Hypothalami were collected and processed for mRNA and peptide analysis, as in Exp. 1.
Exp. 3: NPY Y1 Receptor Antagonism in Juvenile Monkeys.
Seven juvenile monkeys were castrated at 14–16 mo of age and implanted with i.v. catheters and i.c.v. cannulae. To use the pituitary as a bioassay for endogenous GnRH discharges, the sensitivity of the gonadotrophs to GnRH stimulation was heightened by an intermittent i.v. infusion of GnRH (3). The NPY Y1 receptor antagonist, 1229U91 (20, 21), was studied at two doses; 100 and 300 μg injected as i.c.v. boli in 100 μl of aCSF followed by a 400-μl flush with aCSF. Blood samples were withdrawn by the i.v. catheter before and after administration of the drug to measure changes in circulating LH concentrations. The high dose of the Y1 antagonist was also studied in the presence of a GnRH receptor antagonist. For this purpose, acyline (200 μg/day) was injected s.c. for 3 days before i.c.v. injection of the Y1 antagonist. The Y1 antagonist (3,500 μg) was also administered i.v. to three of the juveniles.
Four animals were used to study the action of NPY on hypothalamic GnRH release. For this purpose, NPY was injected as a single i.c.v. bolus of 100–200 μg: a dose that was first established to modulate LH release in two agonadal postpubertal males (see Results). The action of the Y1 antagonist (300 μg i.c.v.) was also examined in the postpubertal males. Pharmacological experiments in juveniles were completed before 21 mo of age.
Exp. 4: NPY mRNA-Expressing Neurons in Situ.
A second group of eight prepubertal monkeys (15–18 mo of age) were orchidectomized. Four animals (juvenile group) were perfused with 4% paraformaldehyde in 0.1 M PBS (22) within 10 wk of castration. The remaining monkeys (pubertal group) were similarly perfused at 29–35 mo of age after reaugmentation of pulsatile GnRH release had been established, as in Exp 1. Hypothalami were bisected in the sagittal plane and, for this study, one half was postfixed in paraformaldehyde. Then, 25-μm sections of the MBH and POA were cut on a microtome and stored at −20°C in cryoprotectant (22).
Results
Exp. 1.
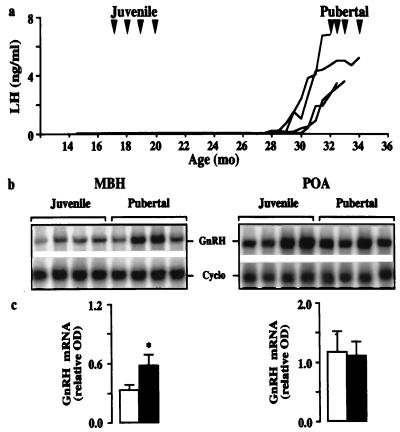
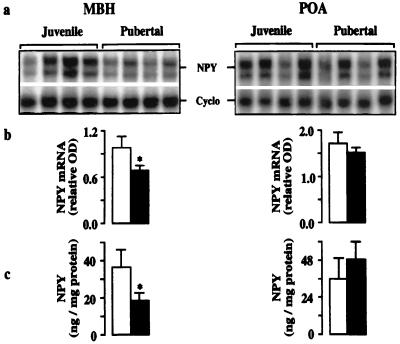
In agonadal male monkeys, the mean level of the mRNA encoding GnRH in the MBH of pubertal animals was 75% greater than that in juveniles (Fig. 1). In contrast, in the POA, developmental changes in this mRNA were not observed. Interestingly, the pubertal amplification in hypothalamic GnRH gene expression in agonadal males was associated with a 30% decrease in levels of the mRNA encoding NPY in the MBH, and this developmental change in NPY gene expression was paralleled by that in NPY peptide content (Fig. 2). Changes in NPY mRNA and peptide levels during the juvenile–pubertal transition were not observed in the POA (Fig. 2).
Figure 1.
Hypothalamic GnRH release and gene expression during the juvenile–pubertal transition. (a) Peripubertal changes in GnRH pulse generator activity, as reflected by LH concentrations in eight agonadal male monkeys. Arrowheads indicate ages at which the monkeys where studied; four were juvenile and four were pubertal. (b) Autoradiograms of GnRH and Cyclo mRNAs in MBH (Left) and POA (Right) hybridized to 32P-labeled antisense probes. The left- and right-hand four lanes of each gel are from juvenile and pubertal monkeys, respectively. Mean ± SD integrated OD for Cyclo: MBH 18,341 ± 2,018; 19,740 ± 1,943; POA 23,413 ± 2,431; 24,734 ± 2,214 (juvenile and pubertal, respectively). (c) Corresponding values for the mean ± SD relative OD of the GnRH mRNA in juvenile (open bar, n = 4) and pubertal monkeys (filled bar, n = 4). *, P < 0.05.
Figure 2.
NPY mRNA and peptide levels in MBH (Left) and POA (Right) of juvenile and pubertal agonadal male monkeys. (a) Autoradiograms of NPY and Cyclo mRNAs hybridized to 32P-labeled antisense probes generated from rat cDNAs. Use of an antisense probe transcribed from hNPY cDNA in additional RNase protection assays of pools containing equal amounts of RNA from each hypothalamic region yielded the same result (not shown). (b) Corresponding relative OD (mean ± SD) of the NPY mRNAs in juvenile (open bar, n = 4) and pubertal monkeys (filled bar, n = 4). (c) NPY peptide content (mean ± SD, n = 4 in each group). *, P < 0.05.
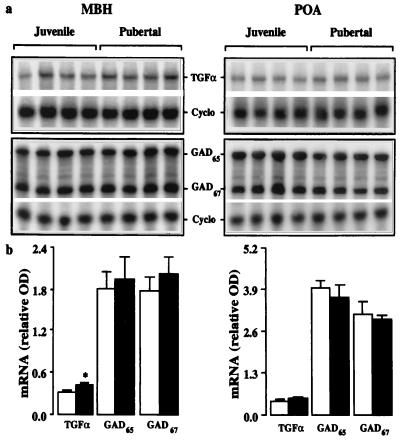
A significant 30% increase in levels of the mRNA encoding TGFα was also observed in the pubertal monkeys when compared with juveniles and, as with changes in GnRH and NPY gene expression, this was observed only in the MBH (Fig. 3). Levels of the mRNAs encoding GAD65 and GAD67 in the juvenile and pubertal animals were indistinguishable in both the MBH and POA (Fig. 3).
Figure 3.
TGFα, GAD65, and GAD67 mRNA levels in MBH (Left) and POA (Right) of juvenile and pubertal monkeys. (a) Autoradiograms of TGFα, GAD65, GAD67, and Cyclo mRNAs hybridized to 32P-labeled antisense probes. (b) Corresponding values for the mean ± SD relative OD of the TGFα, GAD65, and GAD67 mRNA in juvenile (open bar, n = 4) and pubertal monkeys (filled bar, n = 4). *, P < 0.05.
GnRH peptide content (mean ± SD) in the MBH and POA of juvenile monkeys (0.96 ± 0.12 and 0.74 ± 0.16 ng/mg protein, respectively) and pubertal monkeys (0.76 ± 0.11 and 0.72 ± 0.11 ng/mg protein) did not show developmental differences.
Exp. 2.
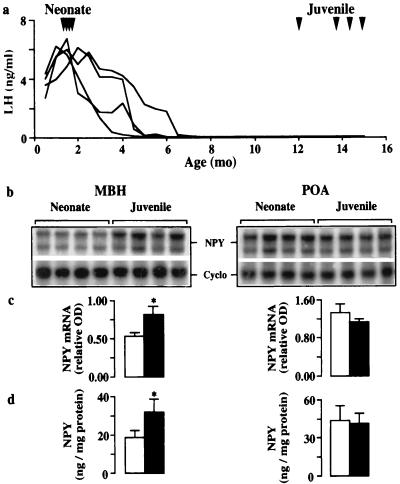
During the neonatal–juvenile transition, NPY mRNA levels and peptide contents in the MBH of the neonates were 35–40% lower than those in juveniles, and, moreover, these developmental changes in NPY gene and protein expression were again restricted to this region of the hypothalamus (Fig. 4). It should also be noted that the mean NPY mRNA level and peptide content in the MBH and POA of the juvenile animals used to study the neonatal–juvenile transition were similar to those observed in the juvenile group used for Exp 1.
Figure 4.
Hypothalamic GnRH release and NPY expression during the neonatal–juvenile transition. (a) GnRH pulse generator activity as reflected by LH concentrations in eight agonadal monkeys. Arrowheads indicate ages at which the monkeys were studied; four were neonatal and four were juvenile. (b) Autoradiograms of NPY and Cyclo mRNAs in MBH (Left) and POA (Right) hybridized to 32P-labeled antisense probes generated from rat cDNAs. Use of an antisense probe generated from hNPY cDNA in additional RNase protection assays of pools containing equal amounts of RNA from each hypothalamic region at each developmental stage yielded the same result (not shown). Mean ± SD integrated OD for Cyclo: MBH 18,430 ± 1,947; 18,160 ± 1,552; POA 18,940 ± 1,817; 20,520 ± 1,983 (neonate and juvenile, respectively). (c) Corresponding relative OD of the NPY mRNAs in neonatal (open bar, n = 4) and pubertal monkeys (filled bar, n = 4). (d) NPY peptide content (mean ± SD, n = 4 in each group). NPY peptide levels may be compared with values in Fig. 2 because they were obtained in a common assay. *, P < 0.05.
Exp. 3.
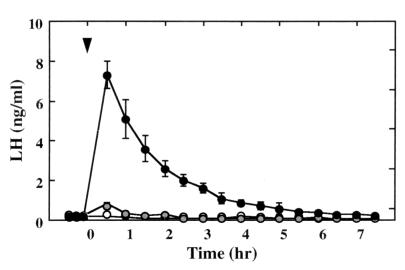
The impact of NPY receptor antagonism on hypothalamic GnRH release in juveniles was assessed indirectly by using the LH response of the pituitary previously sensitized to the releasing factor. Intracerebroventricular injection of 300 μg of 1229U91, an NPY Y1 receptor antagonist, elicited an immediate and robust discharge of LH (Fig. 5), which was abolished by prior treatment with the GnRH receptor antagonist acyline (not shown). Intravenous administration of 1229U91, at doses at least 10 times greater than those administered centrally, failed to elicit LH release (not shown).
Figure 5.
Abrupt increments in LH concentrations (mean ± SD) elicited in response to lateral i.c.v. injection of 100 or 300 μg ( and ●, respectively) of the NPY Y1 receptor antagonist, 1229U91, at time 0 (arrowhead) in four juvenile monkeys with pituitaries sensitized to GnRH. Open data points show response to vehicle alone. The LH concentrations at 30 min after both doses of the Y1 antagonist were significantly different from vehicle.
Intracerebroventricular administration of 100–200 μg of NPY to juveniles failed to elicit GnRH release (not shown). Similar doses of NPY, however, interrupted pulsatile LH release in the two postpubertal animals (not shown). Additionally, i.c.v. administration of the Y1 antagonist to the postpubertal monkeys did not influence spontaneous pulsatile LH release (not shown).
Exp. 4.
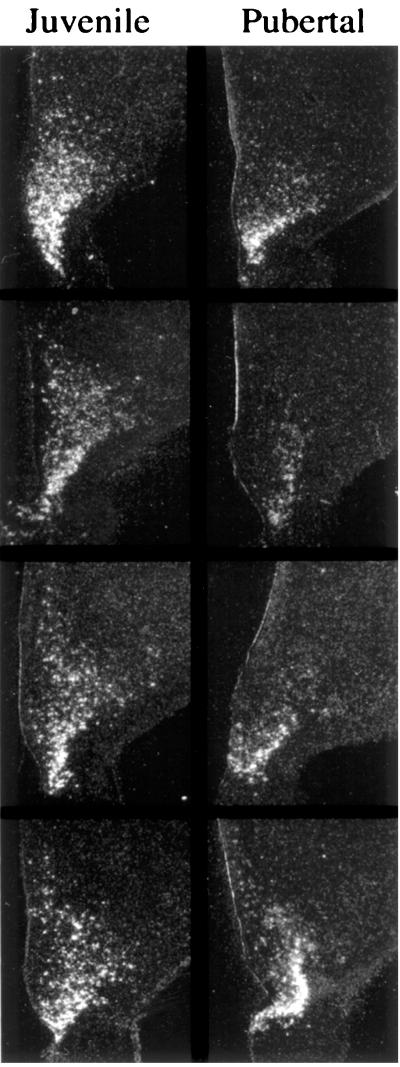
In both juvenile and pubertal monkeys, NPY mRNA-expressing cells were observed concentrated in the region of the arcuate nucleus and distributed diffusely in more dorsal regions of the MBH (Fig. 6). In situ NPY mRNA expression in nonarcuate regions of the juvenile MBH appeared to be greater than that in pubertal monkeys (Fig. 6), although this difference was not quantitated.
Figure 6.
Dark-field photomicrographs of emulsion-dipped hemicoronal sections (25 μm) taken through the midtuberal region of the MBH of four juvenile (Left) and four pubertal (Right) monkeys and hybridized with a 33P-labeled hNPY riboprobe. The ependymal lining of the third ventricle is visible on the left of each section. NPY mRNA expressing neurons are observed in the region of the arcuate nucleus in all animals. Labeling in areas dorsal to the arcuate region was more pronounced in juveniles. Labeling was absent in sections hybridized with sense riboprobe (not shown). (×8.)
Discussion
The present finding of a pubertal increase in the hypothalamic level of the mRNA encoding GnRH in the agonadal male monkey was perhaps to be anticipated because it is well established that an elevated release of the decapeptide occurs at this stage of development in the castrate situation (2, 3). Such a temporal pattern of GnRH gene expression, however, has not been observed in intact monkeys (7, 9). Presumably, in the presence of the gonad, the developmentally programmed drive to increase GnRH gene expression in the MBH is limited by the concomitant inhibitory action of enhanced gonadal hormone secretion. The foregoing view is supported by the finding that, in the adult monkey, orchidectomy results in an increase in GnRH mRNA in the MBH (16), the magnitude of which is similar to the amplification in GnRH gene expression observed in the present study during the peripubertal transition. In contrast to mRNA levels, hypothalamic GnRH peptide content did not change during the juvenile–pubertal transition, as has been previously reported (23).
The finding of an inverse correlation between increasing hypothalamic GnRH gene expression and release on the one hand and diminishing NPY mRNA and peptide levels on the other during the juvenile–pubertal transition in agonadal males was particularly intriguing to us, because, in the adult female monkey, i.c.v. administration of this neuropeptide had been reported to inhibit pulsatile GnRH release (8). Together, these observations raised the possibility that an increased NPY tone in the MBH of the juvenile may play a role in restraining the GnRH pulse generator during this stage of development, an idea that was reinforced by our finding that the inhibitory action of NPY on pulsatile GnRH release postpubertally is also demonstrable in the male. We reasoned that, if there was merit to this notion, it was to be predicted that NPY mRNA and peptide levels in the hypothalamus of neonatal monkeys would be low because, at the early stage of postnatal development, the GnRH pulse generator has yet to be brought into check. This proved to be the case, with changes in hypothalamic NPY gene and protein expression during the neonatal–juvenile transition again being restricted to the MBH.
On the other hand, although findings by others (4, 5) have indicated that GABA may be an important neurochemical component of the restraint imposed on pulsatile GnRH release in the juvenile female monkey, in the present study, levels of the mRNAs encoding the GABA-synthesizing enzymes, GAD65 and GAD67, were indistinguishable in the juvenile and pubertal groups. This result confirms an earlier study employing in situ hybridization (24), and together they indicate that, in the male monkey, the lifting of the prepubertal brake on GnRH release is not the consequence of a decrease in GAD gene expression.
Similarly, TGFα has been implicated in the pubertal amplification of GnRH release in the female monkey (7), but, in the present study, the juvenile–pubertal increase in levels of the mRNA encoding this glial growth factor was less striking than that reported for the female. Also, the recent finding that GnRH neurons make frequent synaptoid contacts with astrocytes in the MBH of the monkey raises the possibility that the pubertal increase in TGFα gene expression may be the consequence rather than a cause of increased GnRH neuronal activity (22). Moreover, in contrast to the female monkey (7), hypothalamic TGFα mRNA levels in agonadal males do not change during the neonatal–juvenile transition (M.E.M., A.S., and T.M.P., unpublished observations).
For the above-mentioned reasons, NPY became the focus of our attention, and we next determined whether the elevated NPY tone of the juvenile MBH is instrumental in restraining the GnRH pulse generator during this phase of development. Intracerebroventricular injection of the NPY Y1 receptor antagonist, 1229U91, elicited a dose-dependent discharge of GnRH as reflected by the pituitary LH response. Although 1229U91 is also an agonist at the NPY Y4 receptor (21), the ability of this compound to elicit precocious GnRH release from the hypothalamus of juveniles was apparently unrelated to the latter action because i.c.v. administration of NPY to juveniles, at doses shown to modulate GnRH release in postpubertal animals, failed to mimic the effect of 1229U91.
In accord with results from RNase protection assay, in situ hybridization revealed that NPY mRNA-expressing cells in the monkey MBH were more noticeable in juveniles than in pubertal animals, and this was particularly apparent for a subset of NPY mRNA-expressing neurons found diffusely distributed in regions dorsal to the arcuate nucleus. In order for NPY neurons in the MBH of the juvenile to restrain pulsatile GnRH release directly, they must send projections either to the median eminence, to interact with GnRH terminals, or to areas such as the ventrohypothalamic tract, where the majority of GnRH perikarya in the primate MBH are located (25). The first possibility seems unlikely because, at the level of the monkey median eminence, NPY is recognized to exert stimulatory actions on GnRH release (8, 26), and, in the present study, i.v. administration of large doses of the Y1 antagonist failed to elicit GnRH release in juveniles.
Although preliminary double-label immunostaining has failed to provide evidence for interactions between GnRH perikarya and NPY terminals in the monkey (27), this issue now requires more detailed analysis in view of the findings reported here. We have previously demonstrated that synaptic input to GnRH perikarya in the MBH, but not the POA, declines in association with the lifting of the prepubertal brake on pulsatile GnRH release (28), and it is therefore tempting to speculate that the synapses lost may be NPYergic. Certainly, postsynaptic inhibition of GnRH neurons is consistent with the view that NPY receptors, including the Y1 subtype, are G-protein-coupled receptors (29), which, on activation, lead to membrane hyperpolarization (30).
The possibility that NPY neurons may also indirectly restrain GnRH release in the juvenile cannot be excluded. Here, it is to be noted that the precocious GnRH discharge elicited by Y1 receptor antagonism was reminiscent of the explosive episodes of GnRH secretion that are readily evoked at this stage of development in response to intermittent activation of hypothalamic glutamate receptors (10, 30). Thus, the prepubertal restraint on GnRH release may be mediated, in part, by NPY inhibition of glutamatergic interneurons. In either case, it seems reasonable to propose that the waning in hypothalamic NPY tone at the termination of the juvenile phase may be temporarily coupled to a structural remodeling in a subset of NPY neurons in the MBH. Such a view is consistent with the present result that the Y1 receptor antagonist failed to elicit GnRH release in two postpubertal monkeys, and with the notion that the MBH of the pubertal monkey remains plastic as reflected by the expression of embryonic neural cell adhesion molecule in this hypothalamic area (32).
The finding that developmental changes in hypothalamic NPY gene and peptide expression were restricted, without exception, to the MBH is worthy of emphasis. This is because the MBH is considered to contain the fundamental neural components underlying the onset of puberty in the monkey (33, 34), and, therefore, this anatomical specificity provides further, albeit circumstantial, support for the importance of NPY in dictating the ontogeny of pulsatile GnRH release.
The foregoing considerations lead us to propose that, in male primates, a removal of an inhibitory NPY input to hypothalamic GnRH neurons at the end of the juvenile phase of development reestablishes the hypophysiotropic drive to the pituitary–gonadal axis and leads to the onset of puberty. The overall pattern of GnRH pulse generator activity during postnatal development in male and female primates is qualitatively similar (2), and, therefore, it is likely that the prepubertal activation of NPY neurons observed in the male MBH will also be operative in the female. The hypothalamic restraint imposed on GnRH release in the prepubertal female, however, is less intense than that in the male (2), and it is reasonable to predict that the NPY brake in the female will be correspondingly weaker. On the other hand, the restraint imposed by the gonad on gonadotropin release in the premenarcheal monkey is relatively robust (35), whereas testicular feedback at a corresponding stage of development is at best trivial (2). Thus, the data from the intact female demonstrating a pivotal role for GABA as an inhibitor of GnRH release before puberty (4, 5), and those generated by the present study implicating NPY in this regard may be reconciled by proposing that gonadal restraint on prepubertal GnRH release is mediated by GABA inhibition. Accordingly, the MBH of the prepubertal female would exhibit a marked GABA tone, which, when antagonized, would lead as previously established to precocious GnRH release (4, 5).
In addition to NPY neurons providing the brake that is imposed on the GnRH pulse generator before the onset of puberty, evidence indicates that NPY also subserves neuronal networks in the primate MBH that facilitate GnRH release after the initiation of puberty. In pubertal and postpubertal female monkeys, but not in premenarcheal subjects, NPY infusion in the region of the median eminence stimulates GnRH release, and infusion of NPY antibody into this hypothalamic area suppresses GnRH discharges (36). The emergence of this stimulatory action of NPY at puberty is associated with an amplification in pulsatile release of the neuropeptide in the median eminence, which is temporally coupled to that of GnRH (36). We therefore speculate that two functionally distinct subsets of GnRH regulating NPY neurons must reside within the primate MBH. One subset is developmentally governed and comprises the neurobiological brake that holds the GnRH pulse generator in check during juvenile development; these NPY neurons project away from the median eminence to areas containing GnRH perikarya. The second subset comprises an intrinsic component of the GnRH pulse generating neuronal network; these NPY neurons project to the median eminence, an area devoid of GnRH cell bodies but containing GnRH axons and terminals. Whether the two subsets of NPY perikarya proposed in this model are anatomically distinct remains to be established.
Acknowledgments
We thank J. Balcita, R. Friedman, A. Durrant, D. Bolette, and the Cores of the Center for Research in Reproductive Physiology for technical assistance, and L. Youngju for statistical advice. We thank Dr. R. B. Gibbs for help with cell imaging, and Drs. A. J. Daniels, T. G. Golos, S. R. Ojeda, K-Y. F. Pau, J. L. Roberts, S. L. Sabol, H. G. Spies, R. A. Steiner, E. Terasawa, and H. F. Urbanski for probes, antibodies, and antagonists. This work was supported by National Institutes of Health Grants HD 08610 and 13254.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- Cyclo
cyclophilin
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- GnRH
gonadotropin-releasing hormone
- h
human
- i.c.v.
intracerebroventricular
- MBH
mediobasal hypothalamus
- NPY
neuropeptide Y
- TGF
transforming growth factor
- LH
luteinizing hormone
- POA
preoptic area
Footnotes
Deceased April 13, 2000.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090099697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090099697
References
- 1.Hotchkiss J, Knobil E. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. Vol. 2. New York: Raven; 1994. pp. 711–749. [Google Scholar]
- 2.Plant T M. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. Vol. 2. New York: Raven; 1994. pp. 453–485. [Google Scholar]
- 3.Suter K J, Pohl C R, Plant T M. Endocrinology. 1998;139:2774–2783. doi: 10.1210/endo.139.6.6055. [DOI] [PubMed] [Google Scholar]
- 4.Mitsushima D, Hei D L, Terasawa E. Proc Natl Acad Sci USA. 1994;91:395–399. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsushima D, Marzban F, Luchansky L L, Burich A J, Keen K L, Durning M, Golos T G, Terasawa E. J Neurosci. 1996;16:2563–2573. doi: 10.1523/JNEUROSCI.16-08-02563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojeda S R, Ma Y J, Rage F. In: The Neurobiology of Puberty. Plant T M, Lee P A, editors. Bristol: J. Endocrinol.; 1995. pp. 103–117. [Google Scholar]
- 7.Ma Y J, Costa M E, Ojeda S R. Neuroendocrinology. 1994;60:346–359. doi: 10.1159/000126769. [DOI] [PubMed] [Google Scholar]
- 8.Pau K-Y, F, Berria M, Hess D L, Spies H G. J Neuroendocrinol. 1995;7:63–67. doi: 10.1111/j.1365-2826.1995.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 9.Vician L, Adams L A, Clifton D K, Steiner R A. Mol Cell Neurosci. 1991;2:31–38. doi: 10.1016/1044-7431(91)90037-o. [DOI] [PubMed] [Google Scholar]
- 10.Gay V L, Mikuma N, Plant T M. Am J Physiol. 1993;264:E476–E481. doi: 10.1152/ajpendo.1993.264.3.E476. [DOI] [PubMed] [Google Scholar]
- 11.Plant T M. Endocrinology. 1986;119:539–545. doi: 10.1210/endo-119-2-539. [DOI] [PubMed] [Google Scholar]
- 12.Larsen P J, Tang-Christensen M, Stidsen C E, Madsen K, Smith M S, Cameron J L. J Clin Endocrinol Metab. 1999;84:3781–3791. doi: 10.1210/jcem.84.10.5897. [DOI] [PubMed] [Google Scholar]
- 13.Khorram O, Roselli C E, Ellinwood W E, Spies H G. Peptides. 1987;8:159–163. doi: 10.1016/0196-9781(87)90180-x. [DOI] [PubMed] [Google Scholar]
- 14.Sahu A, Crowley W R, Kalra P S, Kalra S P. Brain Res. 1992;573:235–242. doi: 10.1016/0006-8993(92)90768-5. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen D D, Kennedy B P, Ziegler M G, Nett T M. Endocrinology. 1988;123:2916–2921. doi: 10.1210/endo-123-6-2916. [DOI] [PubMed] [Google Scholar]
- 16.El Majdoubi M, Ramaswamy S, Sahu A, Plant T M. J Neuroendocrinol. 2000;12:167–176. doi: 10.1046/j.1365-2826.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi H, Yang H-Y T, Sabol S L. J Biol Chem. 1988;263:6288–6295. [PubMed] [Google Scholar]
- 18.Finn P D, Cunningham M J, Pau K-Y F, Spies H G, Clifton D K, Steiner R A. Endocrinology. 1998;139:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- 19.Danielson P E, Forss-Petter S, Brow M A, Calavetter L, Douglass J, Milner R J, Sutcliffe J G. DNA. 1988;4:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- 20.Daniels A J, Matthews J E, Slepetis R J, Jansen M, Viveros O H, Tadepalli A, Harrington W, Heyer D, Landavazo A, Leban J J, Spaltenstein A. Proc Natl Acad Sci USA. 1995;92:9067–9071. doi: 10.1073/pnas.92.20.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker E M, Babij C K, Balasubramaniam A, Burrier R E, Guzzi M, Hamud F, Mukhopadhyay G, Rudinski M S, Tao Z, Tice M, et al. Eur J Pharmacol. 1998;349:97–105. doi: 10.1016/s0014-2999(98)00171-x. [DOI] [PubMed] [Google Scholar]
- 22.Durrant A R, Plant T M. J Neuroendocrinol. 1999;11:813–821. doi: 10.1046/j.1365-2826.1999.00397.x. [DOI] [PubMed] [Google Scholar]
- 23.Fraser M O, Pohl C R, Plant T M. Biol Reprod. 1989;40:972–980. doi: 10.1095/biolreprod40.5.972. [DOI] [PubMed] [Google Scholar]
- 24.Urbanski H F, Rodrigues S M, Garyfallou V T, Kohama S G. Mol Brain Res. 1998;57:86–91. doi: 10.1016/s0169-328x(98)00070-9. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith P C, Thind K K, Song T, Kim E J, Boggan J E. J Neuroendocrinol. 1990;2:157–168. doi: 10.1111/j.1365-2826.1990.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 26.Woller M J, Terasawa E. Endocrinology. 1991;128:1144–1150. doi: 10.1210/endo-128-2-1144. [DOI] [PubMed] [Google Scholar]
- 27.Thind K K, Boggan J E, Goldsmith P C. Neuroendocrinology. 1993;57:289–298. doi: 10.1159/000126371. [DOI] [PubMed] [Google Scholar]
- 28.Perera A D, Plant T M. J Comp Neurol. 1997;385:71–82. doi: 10.1002/(sici)1096-9861(19970818)385:1<71::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Michel M C, Beck-Sickinger A, Cox H, Doods H N, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 30.Sun L, Philipson L H, Miller R J. J Pharmacol Exp Ther. 1998;284:625–632. [PubMed] [Google Scholar]
- 31.Gay V L, Plant T M. Endocrinology. 1987;119:539–545. [Google Scholar]
- 32.Perera A D, Lagenaur C F, Plant T M. Endocrinology. 1993;133:2729–2735. doi: 10.1210/endo.133.6.7694845. [DOI] [PubMed] [Google Scholar]
- 33.Norman R L, Spies H G. Endocrinology. 1981;108:1723–1729. doi: 10.1210/endo-108-5-1723. [DOI] [PubMed] [Google Scholar]
- 34.Krey L C, Hess D L, Butler W R, Espinosa-Campos J, Lu K-H, Piva F, Plant T M, Knobil E. Endocrinology. 1981;108:1944–1948. doi: 10.1210/endo-108-5-1944. [DOI] [PubMed] [Google Scholar]
- 35.Pohl C R, deRidder C M, Plant T M. J Clin Endocrinol Metab. 1995;80:2094–2101. doi: 10.1210/jcem.80.7.7608261. [DOI] [PubMed] [Google Scholar]
- 36.Gore A C, Mitsushima D, Terasawa E. Neuroendocrinology. 1993;58:22–34. doi: 10.1159/000126508. [DOI] [PubMed] [Google Scholar]