Abstract
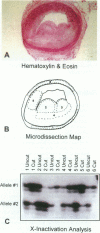
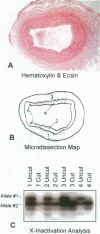
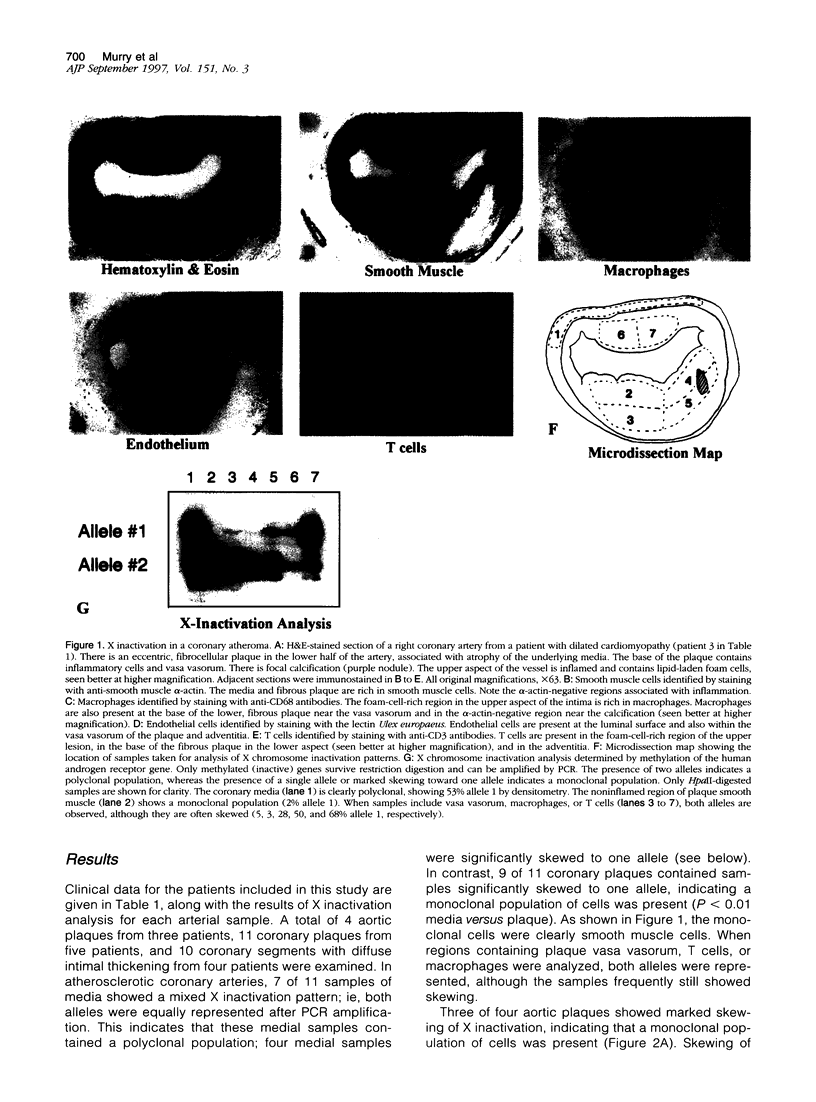
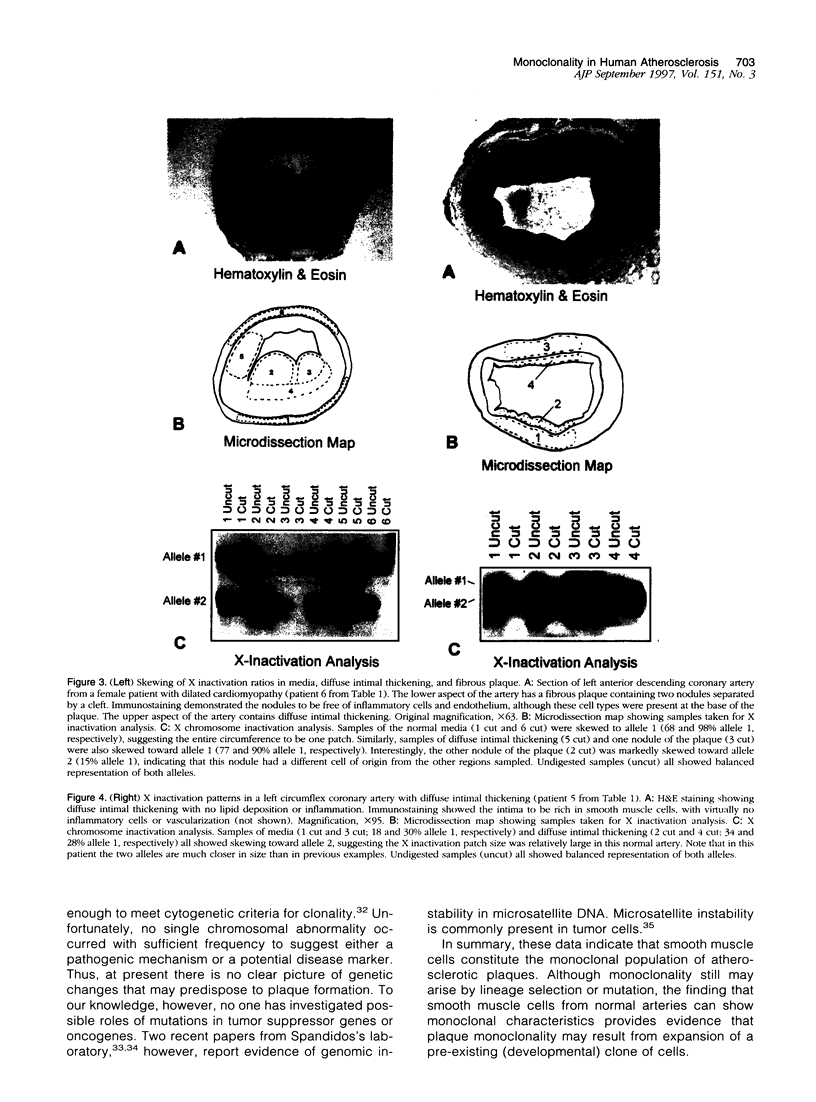
Atherosclerotic plaques contain a large monoclonal population of cells. Monoclonality could arise by somatic mutation, selection of a pre-existing lineage, or expansion of a pre-existing (developmental) clone. To determine the monoclonal cell type in plaque and learn when monoclonality arises, we studied X chromosome inactivation patterns using methylation of the X-linked human androgen receptor gene. Assays based on polymerase chain reaction were performed on samples of known cellular composition, microdissected from histological sections of human arteries. In atherosclerotic vessels, the majority of medial samples (7/11 coronary and 2/3 aortic) showed balanced (paternal and maternal) patterns of X inactivation, indicating polyclonality. In contrast, most samples of plaque smooth muscle cells showed a single pattern of X inactivation (3/4 aortic plaques and 9/11 coronary plaques; P < 0.01 versus media), indicating that plaque smooth muscle cells are monoclonal. Samples of plaque containing inflammatory or endothelial cells showed balanced X inactivation, also demonstrating polyclonality. Multiple plaques from a given patient showed no bias toward one allele, indicating there was no X-linked selection of cells during plaque growth. To determine whether plaques might arise from pre-existing clones (large X inactivation patches), we then studied 10 normal coronaries with diffuse intimal thickening. Six of the ten coronaries showed skewed X inactivation patterns in normal media and intima, suggesting the patch size in normal arteries is surprisingly large. Thus, smooth muscle cells constitute the monoclonal population in atherosclerotic plaques. The finding that normal arteries may have large X inactivation patches raises the possibility that plaque monoclonality may arise by expanding a pre-existing clone of cells rather than generating a new clone by mutation or selection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Zoghbi H. Y., Moseley A. B., Rosenblatt H. M., Belmont J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Alpers C. E., Beckstead J. H. Monocyte/macrophage derived cells in normal and transplanted human kidneys. Clin Immunol Immunopathol. 1985 Aug;36(2):129–140. doi: 10.1016/0090-1229(85)90114-x. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalone R., Granata P., Minelli E., Portentoso P., Giudici A., Righi R., Castelli P., Socrate A., Frigerio B. Cytogenetic analysis reveals clonal proliferation of smooth muscle cells in atherosclerotic plaques. Hum Genet. 1991 Jun;87(2):139–143. doi: 10.1007/BF00204169. [DOI] [PubMed] [Google Scholar]
- Cavallero C., Di Tondo U., Mingazzini P. L., Pesando P. C., Spagnoli L. G. Cell proliferation in the atherosclerotic lesions of cholesterol-fed rabbits. 2. Histological, ultrastructural and radioautographic observations on epinephrine-treated rabbits. Atherosclerosis. 1973 Jan-Feb;17(1):49–62. doi: 10.1016/0021-9150(73)90134-2. [DOI] [PubMed] [Google Scholar]
- Frid M. G., Moiseeva E. P., Stenmark K. R. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res. 1994 Oct;75(4):669–681. doi: 10.1161/01.res.75.4.669. [DOI] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P. Warner-Lambert/Parke-Davis Award Lecture. Viral pathogenesis of atherosclerosis. Impact of molecular mimicry and viral genes. Am J Pathol. 1991 Dec;139(6):1195–1211. [PMC free article] [PubMed] [Google Scholar]
- Hatzistamou J., Kiaris H., Ergazaki M., Spandidos D. A. Loss of heterozygosity and microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996 Aug 5;225(1):186–190. doi: 10.1006/bbrc.1996.1151. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Virtanen I., Kariniemi A. L., Hormia M., Linder E., Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982 Jul;47(1):60–66. [PubMed] [Google Scholar]
- Lemire J. M., Covin C. W., White S., Giachelli C. M., Schwartz S. M. Characterization of cloned aortic smooth muscle cells from young rats. Am J Pathol. 1994 May;144(5):1068–1081. [PMC free article] [PubMed] [Google Scholar]
- Linder D., Gartler S. M. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965 Oct 1;150(3692):67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994 Oct 1;54(19):5059–5063. [PubMed] [Google Scholar]
- Mashal R. D., Lester S. C., Sklar J. Clonal analysis by study of X chromosome inactivation in formalin-fixed paraffin-embedded tissue. Cancer Res. 1993 Oct 1;53(19):4676–4679. [PubMed] [Google Scholar]
- Mikawa T., Fischman D. A. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C. E., Bartosek T., Giachelli C. M., Alpers C. E., Schwartz S. M. Platelet-derived growth factor-A mRNA expression in fetal, normal adult, and atherosclerotic human aortas. Analysis by competitive polymerase chain reaction. Circulation. 1996 Mar 15;93(6):1095–1106. doi: 10.1161/01.cir.93.6.1095. [DOI] [PubMed] [Google Scholar]
- O'Brien E. R., Alpers C. E., Stewart D. K., Ferguson M., Tran N., Gordon D., Benditt E. P., Hinohara T., Simpson J. B., Schwartz S. M. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993 Aug;73(2):223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Heptinstall R. H. Clonal mapping of the human aorta. Relationship of monoclonal characteristics, lesion thickness, and age in normal intima and atherosclerotic lesions. Am J Pathol. 1987 Jan;126(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solex K., Heptinstall R. H. Clonal markers in the study of the origin and growth of human atherosclerotic lesions. Circ Res. 1978 Jul;43(1):10–18. doi: 10.1161/01.res.43.1.10. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solez K., Heptinstall R. H. Clonal characteristics of cutaneous scars and implications for atherogenesis. Am J Pathol. 1981 Jan;102(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J., Solez K., Heptinstall R. H. Monoclonal characteristics of organising arterial thrombi: Significance in the origin and growth of human atherosclerotic plaques. Lancet. 1979 Jan 6;1(8106):7–11. doi: 10.1016/s0140-6736(79)90453-7. [DOI] [PubMed] [Google Scholar]
- Penn A., Garte S. J., Warren L., Nesta D., Mindich B. Transforming gene in human atherosclerotic plaque DNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7951–7955. doi: 10.1073/pnas.83.20.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A., Snyder C. Arteriosclerotic plaque development is 'promoted' by polynuclear aromatic hydrocarbons. Carcinogenesis. 1988 Dec;9(12):2185–2189. doi: 10.1093/carcin/9.12.2185. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Majesky M. W., Murry C. E. The intima: development and monoclonal responses to injury. Atherosclerosis. 1995 Dec;118 (Suppl):S125–S140. [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli L. G., Villaschi S., Neri L., Palmieri G., Taurino M., Faraglia V., Fiorani P. Autoradiographic studies of the smooth muscle cells in human arteries. Paroi Arterielle. 1981;7(3):107–112. [PubMed] [Google Scholar]
- Spandidos D. A., Ergazaki M., Arvanitis D., Kiaris H. Microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996 Mar 7;220(1):137–140. doi: 10.1006/bbrc.1996.0370. [DOI] [PubMed] [Google Scholar]
- Stemme S., Rymo L., Hansson G. K. Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest. 1991 Dec;65(6):654–660. [PubMed] [Google Scholar]
- Thomas W. A., Lee K. T., Kim D. N. Cell population kinetics in atherogenesis. Cell births and losses in intimal cell mass-derived lesions in the abdominal aorta of swine. Ann N Y Acad Sci. 1985;454:305–315. doi: 10.1111/j.1749-6632.1985.tb11870.x. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Reiner J. M., Florentin R. A., Scott R. F. Population dynamics of arterial cells during atherogenesis. VIII. Separation of the roles of injury and growth stimulation in early aortic atherogenesis in swine originating in pre-existing intimal smooth muscle cell masses. Exp Mol Pathol. 1979 Aug;31(1):124–144. doi: 10.1016/0014-4800(79)90013-3. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Reiner J. M., Janakidevi K., Florentin R. A., Lee K. T. Population dynamics of arterial cells during atherogenesis. X. Study of monotypism in atherosclerotic lesions of black women heterozygous for glucose-6-phosphate dehydrogenase (G-6-PD). Exp Mol Pathol. 1979 Dec;31(3):367–386. doi: 10.1016/0014-4800(79)90038-8. [DOI] [PubMed] [Google Scholar]
- Warnke R. A., Pulford K. A., Pallesen G., Ralfkiaer E., Brown D. C., Gatter K. C., Mason D. Y. Diagnosis of myelomonocytic and macrophage neoplasms in routinely processed tissue biopsies with monoclonal antibody KP1. Am J Pathol. 1989 Dec;135(6):1089–1095. [PMC free article] [PubMed] [Google Scholar]
- Wohrley J. D., Frid M. G., Moiseeva E. P., Orton E. C., Belknap J. K., Stenmark K. R. Hypoxia selectively induces proliferation in a specific subpopulation of smooth muscle cells in the bovine neonatal pulmonary arterial media. J Clin Invest. 1995 Jul;96(1):273–281. doi: 10.1172/JCI118031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew P. R., Rajavashisth T. B., Forrester J., Barath P., Lusis A. J. NIH3T3 transforming gene not a general feature of atherosclerotic plaque DNA (atherosclerosis/oncogene/NIH3T3 transfection assay). Biochem Biophys Res Commun. 1989 Dec 29;165(3):1067–1071. doi: 10.1016/0006-291x(89)92710-1. [DOI] [PubMed] [Google Scholar]