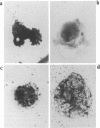
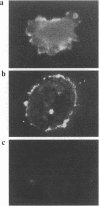
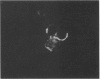
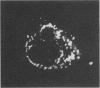
Abstract
Ryanodine receptors (RyRs) reside in microsomal membranes where they gate Ca2+ release in response to changes in the cytosolic Ca2+ concentration. In the osteoclast, a divalent cation sensor, the Ca2+ receptor (CaR), located within the cell's plasma membrane, monitors changes in the extracellular Ca2+ concentration. Here we show that a RyR-like molecule is a functional component of this receptor. We have demonstrated that [3H] ryanodine specifically binds to freshly isolated rat osteoclasts. The binding was displaced by ryanodine itself, the CaR agonist Ni2+ and the RyR antagonist ruthenium red. The latter also inhibited cytosolic Ca2+ elevations induced by Ni2+. In contrast, the responses to Ni2+ were strongly potentiated by an antiserum Ab129 raised to an epitope located within the channel-forming domain of the type II RyR. The antiserum also stained the surface of intact, unfixed, trypan blue-negative osteoclasts. Serial confocal sections and immunogold scanning electron microscopy confirmed a plasma membrane localization of this staining. Antiserum Ab34 directed to a putatively intracellular RyR epitope expectedly did not stain live osteoclasts nor did it potentiate CaR activation. It did, however, stain fixed, permeabilized cells in a distinctive cytoplasmic pattern. We conclude that an RyR-like molecule resides within the osteoclast plasma membrane and plays in important role in extracellular Ca2+ sensing.
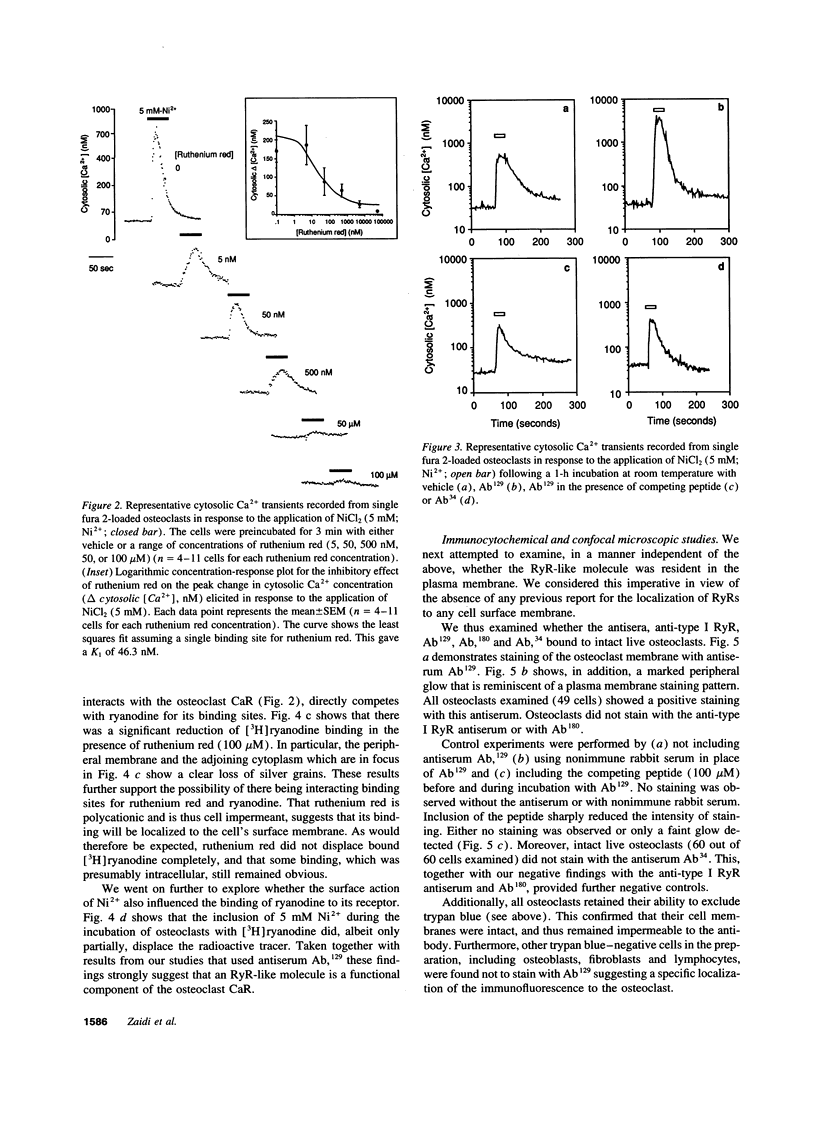
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K., Lai F. A., Liu Q. Y., Rousseau E., Erickson H. P., Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem. 1989 Jan 15;264(2):1329–1335. [PubMed] [Google Scholar]
- Ashley R. H., Williams A. J. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J Gen Physiol. 1990 May;95(5):981–1005. doi: 10.1085/jgp.95.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. Molecular mechanisms of bone resorption by the osteoclast. Anat Rec. 1989 Jun;224(2):317–324. doi: 10.1002/ar.1092240220. [DOI] [PubMed] [Google Scholar]
- Bax B. E., Shankar V. S., Bax C. M., Alam A. S., Zara S., Moonga B. S., Pazianas M., Huang C. L., Zaidi M. Functional consequences of the interaction of Ni2+ with the osteoclast Ca2+ 'receptor'. Exp Physiol. 1993 Jul;78(4):517–529. doi: 10.1113/expphysiol.1993.sp003703. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S., Marshall M. W. Effects of intracellular ruthenium red on excitation-contraction coupling in intact frog skeletal muscle fibres. J Physiol. 1989 Jan;408:617–635. doi: 10.1113/jphysiol.1989.sp017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Brown E. M. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991 Apr;71(2):371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Gamba G., Riccardi D., Lombardi M., Butters R., Kifor O., Sun A., Hediger M. A., Lytton J., Hebert S. C. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993 Dec 9;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Caputo C. Nickel substitution for calcium and the time course of potassium contractures of single muscle fibres. J Muscle Res Cell Motil. 1981 Jun;2(2):167–182. doi: 10.1007/BF00711867. [DOI] [PubMed] [Google Scholar]
- Coronado R., Morrissette J., Sukhareva M., Vaughan D. M. Structure and function of ryanodine receptors. Am J Physiol. 1994 Jun;266(6 Pt 1):C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Csernoch L., Pizarro G., Uribe I., Rodríguez M., Ríos E. Interfering with calcium release suppresses I gamma, the "hump" component of intramembranous charge movement in skeletal muscle. J Gen Physiol. 1991 May;97(5):845–884. doi: 10.1085/jgp.97.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta H. K., MacIntyre I., Zaidi M. The effect of extracellular calcium elevation on morphology and function of isolated rat osteoclasts. Biosci Rep. 1989 Dec;9(6):747–751. doi: 10.1007/BF01114813. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray J. C., Park C. S., Valentine A. N. Calcium and the control of renin secretion. Endocr Rev. 1987 Feb;8(1):53–93. doi: 10.1210/edrv-8-1-53. [DOI] [PubMed] [Google Scholar]
- Fried R. M., Tashjian A. H., Jr Unusual sensitivity of cytosolic free Ca2+ to changes in extracellular Ca2+ in rat C-cells. J Biol Chem. 1986 Jun 15;261(17):7669–7674. [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Dent M., Wickenden C., Xu L., Kumari G., Misra M., Lee H. B., Sar M., Meissner G. Expression of a cardiac Ca(2+)-release channel isoform in mammalian brain. Biochem J. 1992 Dec 1;288(Pt 2):553–564. doi: 10.1042/bj2880553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Meldolesi J., Zallone A. Z., Teti A. Control of cytosolic free calcium in rat and chicken osteoclasts. The role of extracellular calcium and calcitonin. J Biol Chem. 1989 Aug 25;264(24):14342–14347. [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Moonga B. S., Moss D. W., Patchell A., Zaidi M. Intracellular regulation of enzyme secretion from rat osteoclasts and evidence for a functional role in bone resorption. J Physiol. 1990 Oct;429:29–45. doi: 10.1113/jphysiol.1990.sp018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E. F., Scarpa A. Are changes in intracellular free calcium necessary for regulating secretion in parathyroid cells? Ann N Y Acad Sci. 1987;493:542–551. doi: 10.1111/j.1749-6632.1987.tb27239.x. [DOI] [PubMed] [Google Scholar]
- Pazianas M., Zaidi M., Huang C. L., Moonga B. S., Shankar V. S. Voltage sensitivity of the osteoclast calcium receptor. Biochem Biophys Res Commun. 1993 May 14;192(3):1100–1105. doi: 10.1006/bbrc.1993.1530. [DOI] [PubMed] [Google Scholar]
- Shankar V. S., Bax C. M., Bax B. E., Alam A. S., Moonga B. S., Simon B., Pazianas M., Huang C. L., Zaidi M. Activation of the Ca2+ "receptor" on the osteoclast by Ni2+ elicits cytosolic Ca2+ signals: evidence for receptor activation and inactivation, intracellular Ca2+ redistribution, and divalent cation modulation. J Cell Physiol. 1993 Apr;155(1):120–129. doi: 10.1002/jcp.1041550116. [DOI] [PubMed] [Google Scholar]
- Shankar V. S., Pazianas M., Huang C. L., Simon B., Adebanjo O. A., Zaidi M. Caffeine modulates Ca2+ receptor activation in isolated rat osteoclasts and induces intracellular Ca2+ release. Am J Physiol. 1995 Mar;268(3 Pt 2):F447–F454. doi: 10.1152/ajprenal.1995.268.3.F447. [DOI] [PubMed] [Google Scholar]
- Silver I. A., Murrills R. J., Etherington D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988 Apr;175(2):266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Hagiwara S. Membrane depolarization and intracellular Ca2+ increase caused by high external Ca2+ in a rat calcitonin-secreting cell line. J Physiol. 1990 Dec;431:243–267. doi: 10.1113/jphysiol.1990.sp018329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi M., Alam A. S., Huang C. L., Pazianas M., Bax C. M., Bax B. E., Moonga B. S., Bevis P. J., Shankar V. S. Extracellular Ca2+ sensing by the osteoclast. Cell Calcium. 1993 Apr;14(4):271–277. doi: 10.1016/0143-4160(93)90048-b. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Datta H. K., Moonga B. S., MacIntyre I. Evidence that the action of calcitonin on rat osteoclasts is mediated by two G proteins acting via separate post-receptor pathways. J Endocrinol. 1990 Sep;126(3):473–481. doi: 10.1677/joe.0.1260473. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Datta H. K., Patchell A., Moonga B., MacIntyre I. 'Calcium-activated' intracellular calcium elevation: a novel mechanism of osteoclast regulation. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1461–1465. doi: 10.1016/0006-291x(89)91143-1. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Shankar V. S., Bax C. M., Bax B. E., Bevis P. J., Pazianas M., Alam A. S., Moonga B. S., Huang C. L. Linkage of extracellular and intracellular control of cytosolic Ca2+ in rat osteoclasts in the presence of thapsigargin. J Bone Miner Res. 1993 Aug;8(8):961–967. doi: 10.1002/jbmr.5650080809. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Shankar V. S., Towhidul Alam A. S., Moonga B. S., Pazianas M., Huang C. L. Evidence that a ryanodine receptor triggers signal transduction in the osteoclast. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1332–1336. doi: 10.1016/0006-291x(92)91377-3. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Towhidul Alam A. S., Bax C., Shankar V., Bevis P. J., Huang C. L., Pazianas M., Moonga B. S. Cytosolic free calcium measurements in single cells using calcium-sensitive fluorochromes. Methods Mol Biol. 1994;27:279–293. doi: 10.1385/0-89603-250-7:279. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]