Abstract
Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) is a multifunctional oncoprotein. A 30-bp deletion of the 3' end of the LMP1 gene (del-LMP1) has been identified in some EBV isolates. This deleted LMP1 gene encodes a protein, altered on the carboxy terminus, which is thought to have greater oncogenic potential than the wild type. Recently, it was suggested that del-LMP1 plays a role in the development of malignant lymphomas occurring in immunocompromised patients. To further elucidate the role of del-LMP1 in post-transplantation lymphoproliferative disorders (PT-LPDs) we analyzed 58 PT-LPD lesions from 36 heart and kidney organ transplant recipients. Overall, del-LMP1 was detected in 44% of the cases. Four plasmacytic hyperplasias (36%), eight polymorphic B-cell hyperplasias/polymorphic B-cell lymphomas (38%), and five malignant lymphomas/multiple myelomas (71%) exhibited del-LMP1. Two of the three patients displaying disease progression showed wild-type LMP1 gene (w-LMP1) and one showed del-LMP1. LMP1 status remained the same in all three patients during disease progression. In patients undergoing biopsy of multiple separate PT-LPD lesions representing different clonal lymphoid proliferations, LMP1 status was the same in all of the lesions in each patient. Furthermore, although the polyclonal lesions harbor multiple EBV infectious events, they either showed w- or del-LMP1 but not both. Analysis of the tissues without an apparent PT-LPD (peripheral blood, bone marrow, or colon) revealed EBV and LMP1 type identical to that found in the lesions. In conclusion, the presence or absence of del-LMP1 in PT-LPDs does not correlate with the histopathological category or the malignant nature of the lymphoid proliferation. LMP1 status does not change during disease progression and is the same within multiple lesions occurring in the same patient regardless of their clonal relationship. These findings suggest that 1) EBV infection in patients with PT-LPDs occurs with a w- or del-LMP1-type EBV isolate and does not change once a patient acquires the virus and 2) the infection is an early event in the development of PT-LPDs and transformation is induced regardless of the type of LMP1.
Full text
PDF


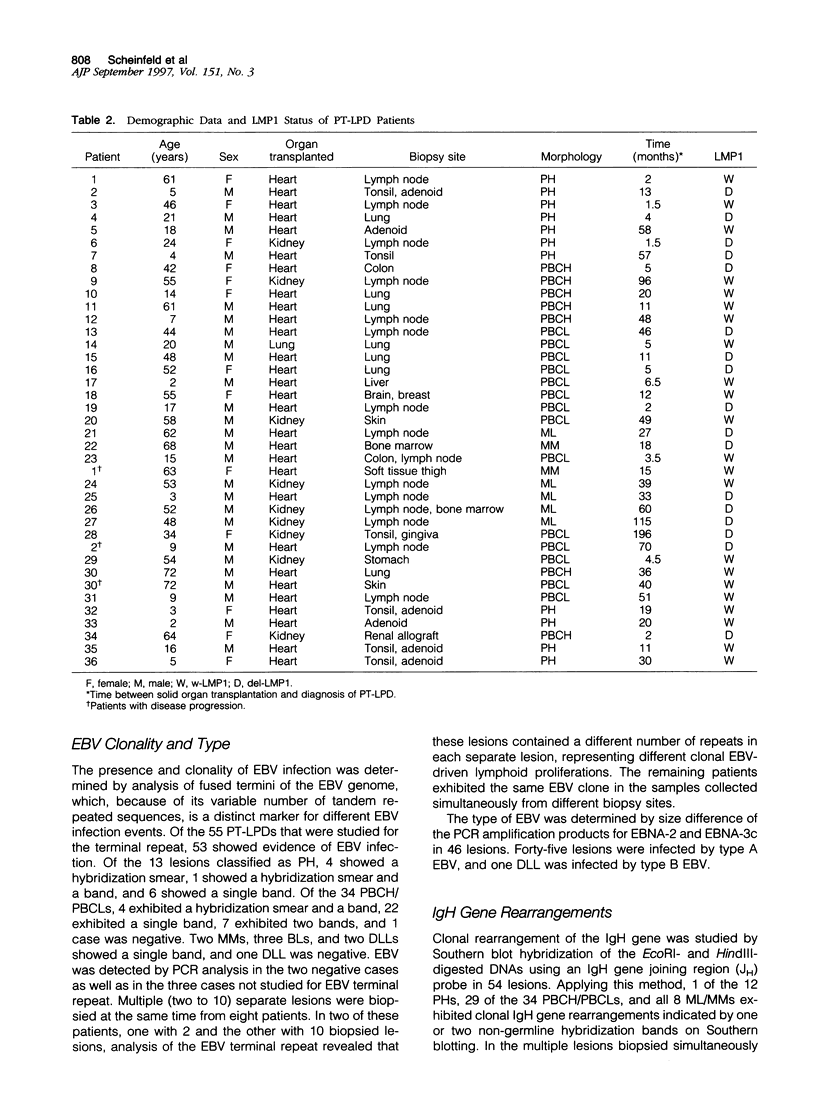
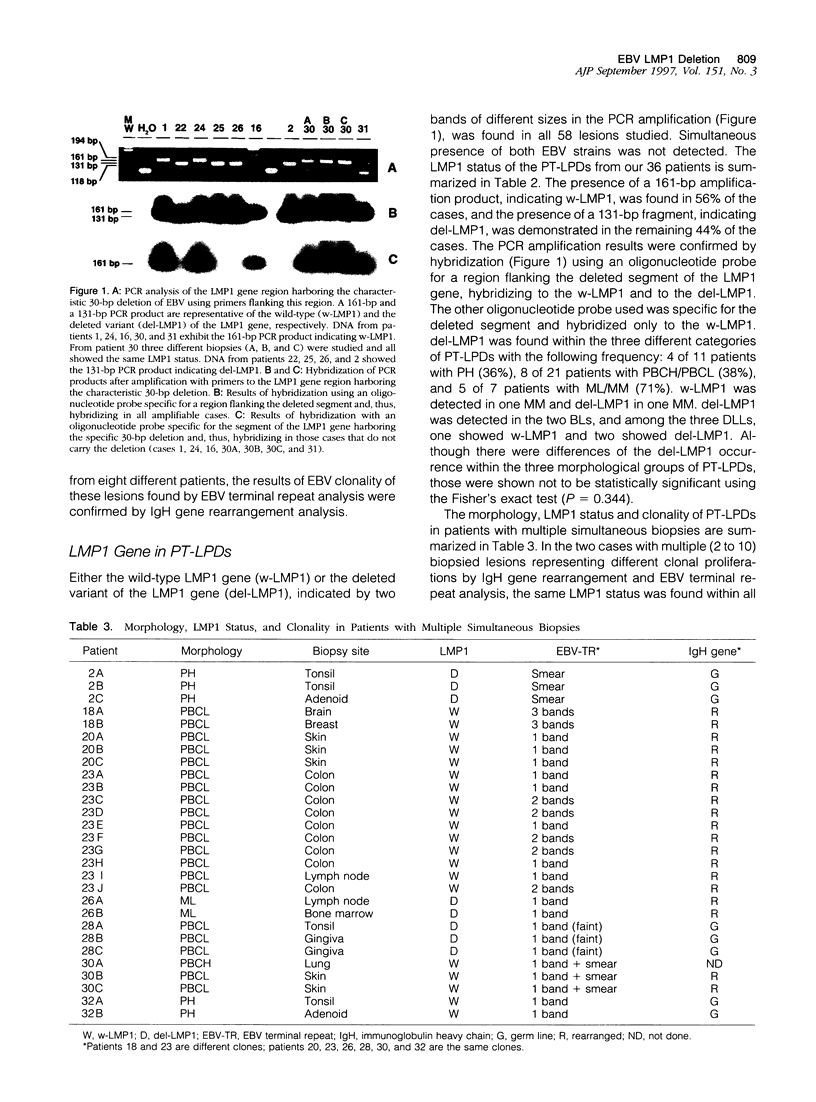



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chadburn A., Cesarman E., Liu Y. F., Addonizio L., Hsu D., Michler R. E., Knowles D. M. Molecular genetic analysis demonstrates that multiple posttransplantation lymphoproliferative disorders occurring in one anatomic site in a single patient represent distinct primary lymphoid neoplasms. Cancer. 1995 Jun 1;75(11):2747–2756. doi: 10.1002/1097-0142(19950601)75:11<2747::aid-cncr2820751119>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Chen M. L., Tsai C. N., Liang C. L., Shu C. H., Huang C. R., Sulitzeanu D., Liu S. T., Chang Y. S. Cloning and characterization of the latent membrane protein (LMP) of a specific Epstein-Barr virus variant derived from the nasopharyngeal carcinoma in the Taiwanese population. Oncogene. 1992 Nov;7(11):2131–2140. [PubMed] [Google Scholar]
- Chen W. G., Chen Y. Y., Bacchi M. M., Bacchi C. E., Alvarenga M., Weiss L. M. Genotyping of Epstein-Barr virus in Brazilian Burkitt's lymphoma and reactive lymphoid tissue. Type A with a high prevalence of deletions within the latent membrane protein gene. Am J Pathol. 1996 Jan;148(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Delecluse H. J., Kremmer E., Rouault J. P., Cour C., Bornkamm G., Berger F. The expression of Epstein-Barr virus latent proteins is related to the pathological features of post-transplant lymphoproliferative disorders. Am J Pathol. 1995 May;146(5):1113–1120. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fennewald S., van Santen V., Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984 Aug;51(2):411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry J. A., Jacobson J. O., Conti D., Delmonico F., Harris N. L. Lymphoproliferative disorders and hematologic malignancies following organ transplantation. Mod Pathol. 1989 Nov;2(6):583–592. [PubMed] [Google Scholar]
- Frank D., Cesarman E., Liu Y. F., Michler R. E., Knowles D. M. Posttransplantation lymphoproliferative disorders frequently contain type A and not type B Epstein-Barr virus. Blood. 1995 Mar 1;85(5):1396–1403. [PubMed] [Google Scholar]
- Hanto D. W., Frizzera G., Purtilo D. T., Sakamoto K., Sullivan J. L., Saemundsen A. K., Klein G., Simmons R. L., Najarian J. S. Clinical spectrum of lymphoproliferative disorders in renal transplant recipients and evidence for the role of Epstein-Barr virus. Cancer Res. 1981 Nov;41(11 Pt 1):4253–4261. [PubMed] [Google Scholar]
- Hanto D. W., Sakamoto K., Purtilo D. T., Simmons R. L., Najarian J. S. The Epstein-Barr virus in the pathogenesis of posttransplant lymphoproliferative disorders. Clinical, pathologic, and virologic correlation. Surgery. 1981 Aug;90(2):204–213. [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Hu L. F., Chen F., Zheng X., Ernberg I., Cao S. L., Christensson B., Klein G., Winberg G. Clonability and tumorigenicity of human epithelial cells expressing the EBV encoded membrane protein LMP1. Oncogene. 1993 Jun;8(6):1575–1583. [PubMed] [Google Scholar]
- Hu L. F., Zabarovsky E. R., Chen F., Cao S. L., Ernberg I., Klein G., Winberg G. Isolation and sequencing of the Epstein-Barr virus BNLF-1 gene (LMP1) from a Chinese nasopharyngeal carcinoma. J Gen Virol. 1991 Oct;72(Pt 10):2399–2409. doi: 10.1099/0022-1317-72-10-2399. [DOI] [PubMed] [Google Scholar]
- Hudson G. S., Farrell P. J., Barrell B. G. Two related but differentially expressed potential membrane proteins encoded by the EcoRI Dhet region of Epstein-Barr virus B95-8. J Virol. 1985 Feb;53(2):528–535. doi: 10.1128/jvi.53.2.528-535.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K. M., Kaye K. M., Kieff E. D. Epstein-Barr virus recombinant molecular genetic analysis of the LMP1 amino-terminal cytoplasmic domain reveals a probable structural role, with no component essential for primary B-lymphocyte growth transformation. J Virol. 1994 Jul;68(7):4369–4376. doi: 10.1128/jvi.68.7.4369-4376.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanim F., Yao Q. Y., Niedobitek G., Sihota S., Rickinson A. B., Young L. S. Analysis of Epstein-Barr virus gene polymorphisms in normal donors and in virus-associated tumors from different geographic locations. Blood. 1996 Nov 1;88(9):3491–3501. [PubMed] [Google Scholar]
- Kingma D. W., Weiss W. B., Jaffe E. S., Kumar S., Frekko K., Raffeld M. Epstein-Barr virus latent membrane protein-1 oncogene deletions: correlations with malignancy in Epstein-Barr virus--associated lymphoproliferative disorders and malignant lymphomas. Blood. 1996 Jul 1;88(1):242–251. [PubMed] [Google Scholar]
- Knecht H., Bachmann E., Brousset P., Rothenberger S., Einsele H., Lestou V. S., Delsol G., Bachmann F., Ambros P. F., Odermatt B. F. Mutational hot spots within the carboxy terminal region of the LMP1 oncogene of Epstein-Barr virus are frequent in lymphoproliferative disorders. Oncogene. 1995 Feb 2;10(3):523–528. [PubMed] [Google Scholar]
- Knecht H., Bachmann E., Brousset P., Sandvej K., Nadal D., Bachmann F., Odermatt B. F., Delsol G., Pallesen G. Deletions within the LMP1 oncogene of Epstein-Barr virus are clustered in Hodgkin's disease and identical to those observed in nasopharyngeal carcinoma. Blood. 1993 Nov 15;82(10):2937–2942. [PubMed] [Google Scholar]
- Knecht H., Bachmann E., Joske D. J., Sahli R., Eméry-Goodman A., Casanova J. L., Zilić M., Bachmann F., Odermatt B. F. Molecular analysis of the LMP (latent membrane protein) oncogene in Hodgkin's disease. Leukemia. 1993 Apr;7(4):580–585. [PubMed] [Google Scholar]
- Knowles D. M., Cesarman E., Chadburn A., Frizzera G., Chen J., Rose E. A., Michler R. E. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995 Jan 15;85(2):552–565. [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw M. T., Hurren L., Evans L., Moss D. J., Cooper D. A., Benson E., Esmore D., Sculley T. B. Expression of B-type Epstein-Barr virus in HIV-infected patients and cardiac transplant recipients. AIDS Res Hum Retroviruses. 1992 Nov;8(11):1869–1874. doi: 10.1089/aid.1992.8.1869. [DOI] [PubMed] [Google Scholar]
- Lin J. C., De B. K., Lin S. C. Rapid and sensitive genotyping of Epstein-Barr virus using single-strand conformation polymorphism analysis of polymerase chain reaction products. J Virol Methods. 1993 Jul;43(2):233–246. doi: 10.1016/0166-0934(93)90079-7. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Lin S. C., De B. K., Chan W. P., Evatt B. L., Chan W. C. Precision of genotyping of Epstein-Barr virus by polymerase chain reaction using three gene loci (EBNA-2, EBNA-3C, and EBER): predominance of type A virus associated with Hodgkin's disease. Blood. 1993 Jun 15;81(12):3372–3381. [PubMed] [Google Scholar]
- Martin J., Sugden B. Transformation by the oncogenic latent membrane protein correlates with its rapid turnover, membrane localization, and cytoskeletal association. J Virol. 1991 Jun;65(6):3246–3258. doi: 10.1128/jvi.65.6.3246-3258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy R. K., Thorley-Lawson D. A. All three domains of the Epstein-Barr virus-encoded latent membrane protein LMP-1 are required for transformation of rat-1 fibroblasts. J Virol. 1993 Mar;67(3):1638–1646. doi: 10.1128/jvi.67.3.1638-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalesnik M. A., Jaffe R., Starzl T. E., Demetris A. J., Porter K., Burnham J. A., Makowka L., Ho M., Locker J. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988 Oct;133(1):173–192. [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Dalla Favera R. Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med. 1985 Sep 1;162(3):1015–1024. doi: 10.1084/jem.162.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Lundgren E. Transient expression of the Epstein-Barr virus LMP1 gene in human primary B cells induces cellular activation and DNA synthesis. Oncogene. 1992 Sep;7(9):1775–1782. [PubMed] [Google Scholar]
- Penn I. Malignant lymphomas in organ transplant recipients. Transplant Proc. 1981 Mar;13(1 Pt 2):736–738. [PubMed] [Google Scholar]
- Purtilo D. T., Strobach R. S., Okano M., Davis J. R. Epstein-Barr virus-associated lymphoproliferative disorders. Lab Invest. 1992 Jul;67(1):5–23. [PubMed] [Google Scholar]
- Raab-Traub N., Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986 Dec 26;47(6):883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Randhawa P. S., Yousem S. A., Paradis I. L., Dauber J. A., Griffith B. P., Locker J. The clinical spectrum, pathology, and clonal analysis of Epstein-Barr virus-associated lymphoproliferative disorders in heart-lung transplant recipients. Am J Clin Pathol. 1989 Aug;92(2):177–185. doi: 10.1093/ajcp/92.2.177. [DOI] [PubMed] [Google Scholar]
- Sample J., Young L., Martin B., Chatman T., Kieff E., Rickinson A., Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990 Sep;64(9):4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvej K., Peh S. C., Andresen B. S., Pallesen G. Identification of potential hot spots in the carboxy-terminal part of the Epstein-Barr virus (EBV) BNLF-1 gene in both malignant and benign EBV-associated diseases: high frequency of a 30-bp deletion in Malaysian and Danish peripheral T-cell lymphomas. Blood. 1994 Dec 15;84(12):4053–4060. [PubMed] [Google Scholar]
- Santón A., Manzanal A. I., Campo E., Bellas C. Deletions in the Epstein-Barr virus latent membrane protein-1 oncogene in Hodgkin's disease. Clin Mol Pathol. 1995 Aug;48(4):M184–M187. doi: 10.1136/mp.48.4.m184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smir B. N., Hauke R. J., Bierman P. J., Gross T. G., d'Amore F., Anderson J. R., Greiner T. C. Molecular epidemiology of deletions and mutations of the latent membrane protein 1 oncogene of the Epstein-Barr virus in posttransplant lymphoproliferative disorders. Lab Invest. 1996 Oct;75(4):575–588. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Nalesnik M. A., Porter K. A., Ho M., Iwatsuki S., Griffith B. P., Rosenthal J. T., Hakala T. R., Shaw B. W., Jr, Hardesty R. L. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984 Mar 17;1(8377):583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen L. J., Costanzo-Nordin M. R., Fisher S. G., O'Sullivan E. J., Johnson M. R., Heroux A. L., Dizikes G. J., Pifarre R., Fisher R. I. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. 1990 Dec 20;323(25):1723–1728. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson A. H., Smith J. L., Hunsicker L. G., Tobacman J., Kapelanski D. P., Johnson M., Wright F. H., Behrendt D. M., Corry R. J. Increased frequency of posttransplant lymphomas in patients treated with cyclosporine, azathioprine, and prednisone. Transplantation. 1989 Feb;47(2):293–296. doi: 10.1097/00007890-198902000-00020. [DOI] [PubMed] [Google Scholar]





