Abstract
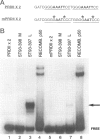
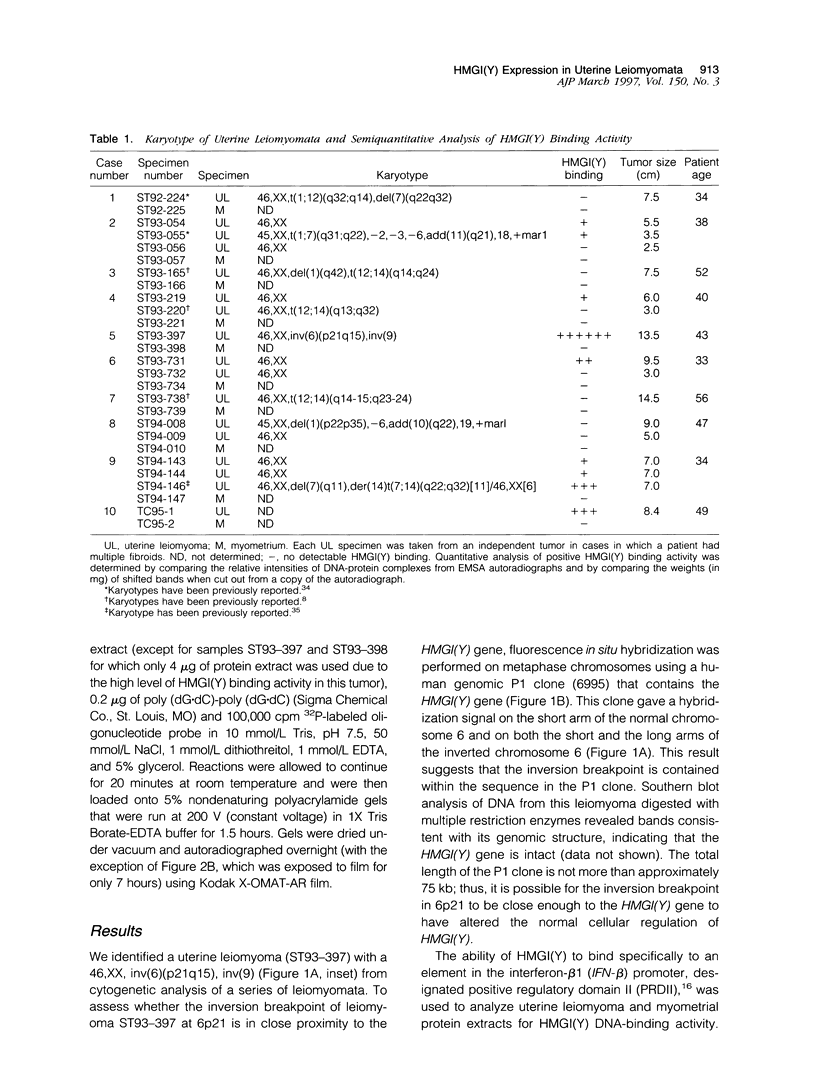
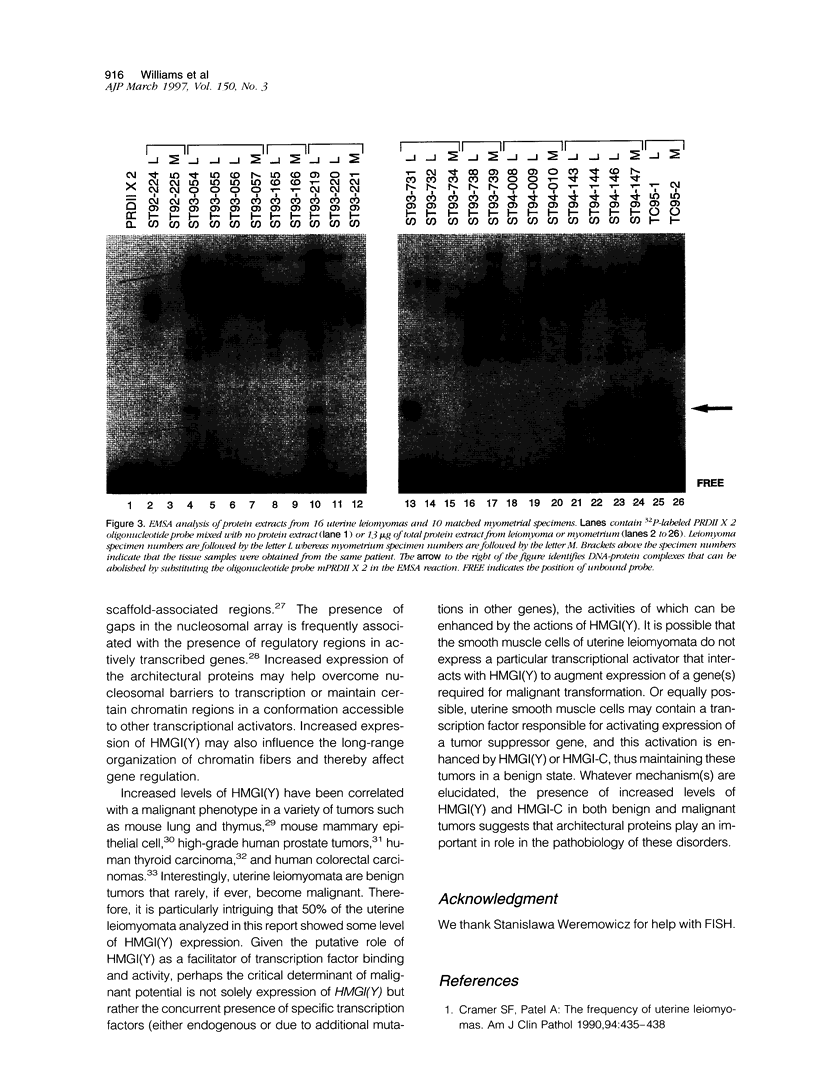
Chromosomal rearrangements involving 6p21 have been observed in uterine leiomyomata and a variety of other benign tumors. The gene for HMGI(Y), a member of the high-mobility group (HMG) family of proteins, has been localized to 6p21. To determine whether rearrangements observed in this area alter HMGI(Y) expression, we analyzed HMGI(Y) DNA-binding activity in protein extracts from uterine leiomyoma and normal myometrium tissues. This report describes a uterine leiomyoma specimen with an inv(6)(p21q15). A genomic P1 clone that contains the HMGI(Y) region of chromosome 6 is found to span the inversion breakpoint by fluorescent in situ hybridization of metaphase chromosomes. Expression of HMGI(Y) protein in this leiomyoma specimen is increased dramatically as compared with the matching normal myometrial tissue. Elevated HMGI(Y) expression was also found in 8 of 16 leiomyomas without cytogenetically detectable chromosome 6p21 aberrations but not in any of the 9 matching myometrial tissues. Analysis of the genetic events involved in the pathobiology of these benign tumors will provide a basis for understanding the process of improper cellular growth and might be important in deciphering the multistep pathway of tumorigenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulkadir S. A., Krishna S., Thanos D., Maniatis T., Strominger J. L., Ono S. J. Functional roles of the transcription factor Oct-2A and the high mobility group protein I/Y in HLA-DRA gene expression. J Exp Med. 1995 Aug 1;182(2):487–500. doi: 10.1084/jem.182.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashar H. R., Fejzo M. S., Tkachenko A., Zhou X., Fletcher J. A., Weremowicz S., Morton C. C., Chada K. Disruption of the architectural factor HMGI-C: DNA-binding AT hook motifs fused in lipomas to distinct transcriptional regulatory domains. Cell. 1995 Jul 14;82(1):57–65. doi: 10.1016/0092-8674(95)90052-7. [DOI] [PubMed] [Google Scholar]
- Chiappetta G., Bandiera A., Berlingieri M. T., Visconti R., Manfioletti G., Battista S., Martinez-Tello F. J., Santoro M., Giancotti V., Fusco A. The expression of the high mobility group HMGI (Y) proteins correlates with the malignant phenotype of human thyroid neoplasias. Oncogene. 1995 Apr 6;10(7):1307–1314. [PubMed] [Google Scholar]
- Chuvpilo S., Schomberg C., Gerwig R., Heinfling A., Reeves R., Grummt F., Serfling E. Multiple closely-linked NFAT/octamer and HMG I(Y) binding sites are part of the interleukin-4 promoter. Nucleic Acids Res. 1993 Dec 11;21(24):5694–5704. doi: 10.1093/nar/21.24.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S. F., Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990 Oct;94(4):435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- Dal Cin P., Moerman P., Deprest J., Brosens I., Van den Berghe H. A new cytogenetic subgroup in uterine leiomyoma is characterized by a deletion of the long arm of chromosome 3. Genes Chromosomes Cancer. 1995 Jul;13(3):219–220. doi: 10.1002/gcc.2870130313. [DOI] [PubMed] [Google Scholar]
- Du W., Thanos D., Maniatis T. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell. 1993 Sep 10;74(5):887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- Falvo J. V., Thanos D., Maniatis T. Reversal of intrinsic DNA bends in the IFN beta gene enhancer by transcription factors and the architectural protein HMG I(Y). Cell. 1995 Dec 29;83(7):1101–1111. doi: 10.1016/0092-8674(95)90137-x. [DOI] [PubMed] [Google Scholar]
- Fedele M., Bandiera A., Chiappetta G., Battista S., Viglietto G., Manfioletti G., Casamassimi A., Santoro M., Giancotti V., Fusco A. Human colorectal carcinomas express high levels of high mobility group HMGI(Y) proteins. Cancer Res. 1996 Apr 15;56(8):1896–1901. [PubMed] [Google Scholar]
- Finco T. S., Baldwin A. S. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995 Sep;3(3):263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- Fletcher J. A., Kozakewich H. P., Hoffer F. A., Lage J. M., Weidner N., Tepper R., Pinkus G. S., Morton C. C., Corson J. M. Diagnostic relevance of clonal cytogenetic aberrations in malignant soft-tissue tumors. N Engl J Med. 1991 Feb 14;324(7):436–442. doi: 10.1056/NEJM199102143240702. [DOI] [PubMed] [Google Scholar]
- Fletcher J. A., Pinkus J. L., Lage J. M., Morton C. C., Pinkus G. S. Clonal 6p21 rearrangement is restricted to the mesenchymal component of an endometrial polyp. Genes Chromosomes Cancer. 1992 Oct;5(3):260–263. doi: 10.1002/gcc.2870050315. [DOI] [PubMed] [Google Scholar]
- Friedmann M., Holth L. T., Zoghbi H. Y., Reeves R. Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res. 1993 Sep 11;21(18):4259–4267. doi: 10.1093/nar/21.18.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti V., Bandiera A., Ciani L., Santoro D., Crane-Robinson C., Goodwin G. H., Boiocchi M., Dolcetti R., Casetta B. High-mobility-group (HMG) proteins and histone H1 subtypes expression in normal and tumor tissues of mouse. Eur J Biochem. 1993 Apr 15;213(2):825–832. doi: 10.1111/j.1432-1033.1993.tb17825.x. [DOI] [PubMed] [Google Scholar]
- Heim S., Mandahl N., Kristoffersson U., Mitelman F., Röser B., Rydholm A., Willén H. Structural chromosome aberrations in a case of angioleiomyoma. Cancer Genet Cytogenet. 1986 Feb 15;20(3-4):325–330. doi: 10.1016/0165-4608(86)90091-9. [DOI] [PubMed] [Google Scholar]
- John S., Reeves R. B., Lin J. X., Child R., Leiden J. M., Thompson C. B., Leonard W. J. Regulation of cell-type-specific interleukin-2 receptor alpha-chain gene expression: potential role of physical interactions between Elf-1, HMG-I(Y), and NF-kappa B family proteins. Mol Cell Biol. 1995 Mar;15(3):1786–1796. doi: 10.1128/mcb.15.3.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak B., Rosigkeit J., Wanschura S., Meyer-Bolte K., Van de Ven W. J., Kayser K., Krieghoff B., Kastendiek H., Bartnitzke S., Bullerdiek J. HMGI-C rearrangements as the molecular basis for the majority of pulmonary chondroid hamartomas: a survey of 30 tumors. Oncogene. 1996 Feb 1;12(3):515–521. [PubMed] [Google Scholar]
- Kim J., Reeves R., Rothman P., Boothby M. The non-histone chromosomal protein HMG-I(Y) contributes to repression of the immunoglobulin heavy chain germ-line epsilon RNA promoter. Eur J Immunol. 1995 Mar;25(3):798–808. doi: 10.1002/eji.1830250326. [DOI] [PubMed] [Google Scholar]
- Lewin B. Chromatin and gene expression: constant questions, but changing answers. Cell. 1994 Nov 4;79(3):397–406. doi: 10.1016/0092-8674(94)90249-6. [DOI] [PubMed] [Google Scholar]
- Nibert M., Heim S. Uterine leiomyoma cytogenetics. Genes Chromosomes Cancer. 1990 May;2(1):3–13. doi: 10.1002/gcc.2870020103. [DOI] [PubMed] [Google Scholar]
- Ozisik Y. Y., Meloni A. M., Altungoz O., Surti U., Sandberg A. A. Translocation (6;10)(p21;q22) in uterine leiomyomas. Cancer Genet Cytogenet. 1995 Feb;79(2):136–138. doi: 10.1016/0165-4608(94)00132-u. [DOI] [PubMed] [Google Scholar]
- Ram T. G., Reeves R., Hosick H. L. Elevated high mobility group-I(Y) gene expression is associated with progressive transformation of mouse mammary epithelial cells. Cancer Res. 1993 Jun 1;53(11):2655–2660. [PubMed] [Google Scholar]
- Rein M. S., Friedman A. J., Barbieri R. L., Pavelka K., Fletcher J. A., Morton C. C. Cytogenetic abnormalities in uterine leiomyomata. Obstet Gynecol. 1991 Jun;77(6):923–926. [PubMed] [Google Scholar]
- Sargent M. S., Weremowicz S., Rein M. S., Morton C. C. Translocations in 7q22 define a critical region in uterine leiomyomata. Cancer Genet Cytogenet. 1994 Oct;77(1):65–68. doi: 10.1016/0165-4608(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Schoenberg Fejzo M., Ashar H. R., Krauter K. S., Powell W. L., Rein M. S., Weremowicz S., Yoon S. J., Kucherlapati R. S., Chada K., Morton C. C. Translocation breakpoints upstream of the HMGIC gene in uterine leiomyomata suggest dysregulation of this gene by a mechanism different from that in lipomas. Genes Chromosomes Cancer. 1996 Sep;17(1):1–6. doi: 10.1002/(SICI)1098-2264(199609)17:1<1::AID-GCC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schoenmakers E. F., Wanschura S., Mols R., Bullerdiek J., Van den Berghe H., Van de Ven W. J. Recurrent rearrangements in the high mobility group protein gene, HMGI-C, in benign mesenchymal tumours. Nat Genet. 1995 Aug;10(4):436–444. doi: 10.1038/ng0895-436. [DOI] [PubMed] [Google Scholar]
- Sreekantaiah C., Leong S. P., Karakousis C. P., McGee D. L., Rappaport W. D., Villar H. V., Neal D., Fleming S., Wankel A., Herrington P. N. Cytogenetic profile of 109 lipomas. Cancer Res. 1991 Jan 1;51(1):422–433. [PubMed] [Google Scholar]
- Tamimi Y., van der Poel H. G., Denyn M. M., Umbas R., Karthaus H. F., Debruyne F. M., Schalken J. A. Increased expression of high mobility group protein I(Y) in high grade prostatic cancer determined by in situ hybridization. Cancer Res. 1993 Nov 15;53(22):5512–5516. [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995 Dec 29;83(7):1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Whitley M. Z., Thanos D., Read M. A., Maniatis T., Collins T. A striking similarity in the organization of the E-selectin and beta interferon gene promoters. Mol Cell Biol. 1994 Oct;14(10):6464–6475. doi: 10.1128/mcb.14.10.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L. D., Farmer A. A., Richmond A. HMGI(Y) and Sp1 in addition to NF-kappa B regulate transcription of the MGSA/GRO alpha gene. Nucleic Acids Res. 1995 Oct 25;23(20):4210–4219. doi: 10.1093/nar/23.20.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Lux M. L., Reeves R., Hudson T. J., Fletcher J. A. HMGI(Y) activation by chromosome 6p21 rearrangements in multilineage mesenchymal cells from pulmonary hamartoma. Am J Pathol. 1997 Mar;150(3):901–910. [PMC free article] [PubMed] [Google Scholar]
- Zhao K., Käs E., Gonzalez E., Laemmli U. K. SAR-dependent mobilization of histone H1 by HMG-I/Y in vitro: HMG-I/Y is enriched in H1-depleted chromatin. EMBO J. 1993 Aug;12(8):3237–3247. doi: 10.1002/j.1460-2075.1993.tb05993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]