Abstract
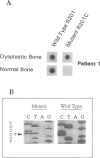
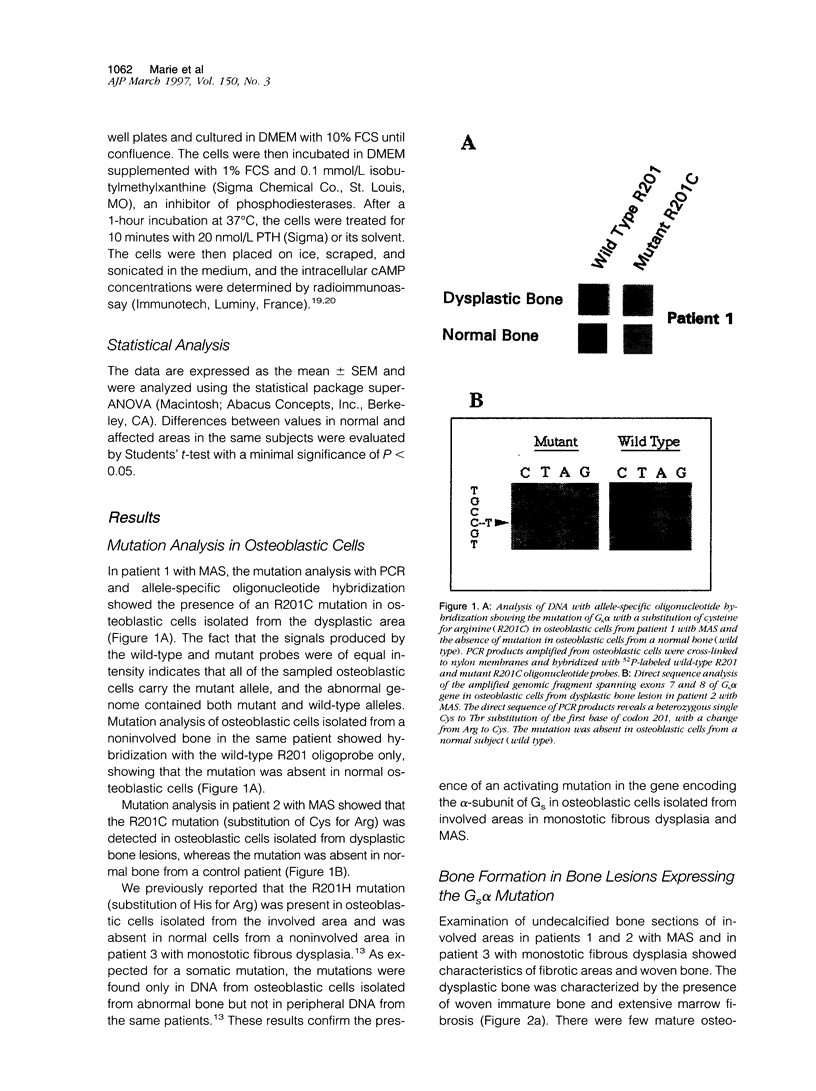
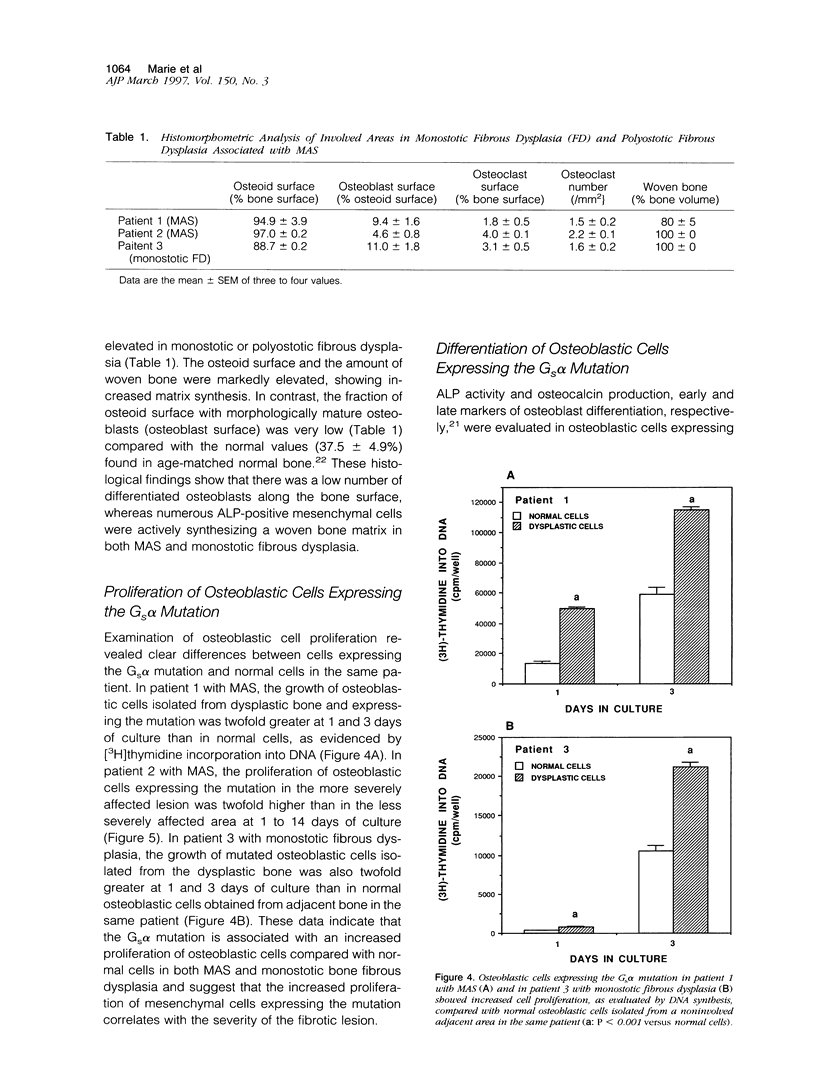
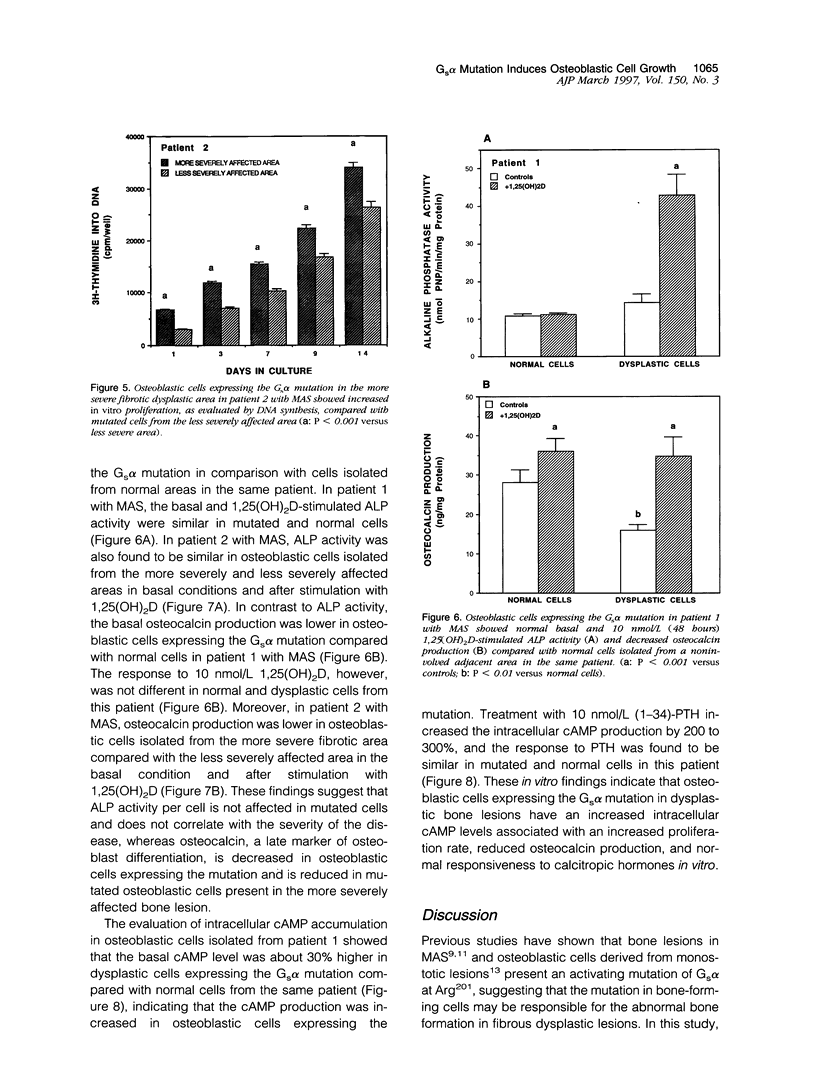
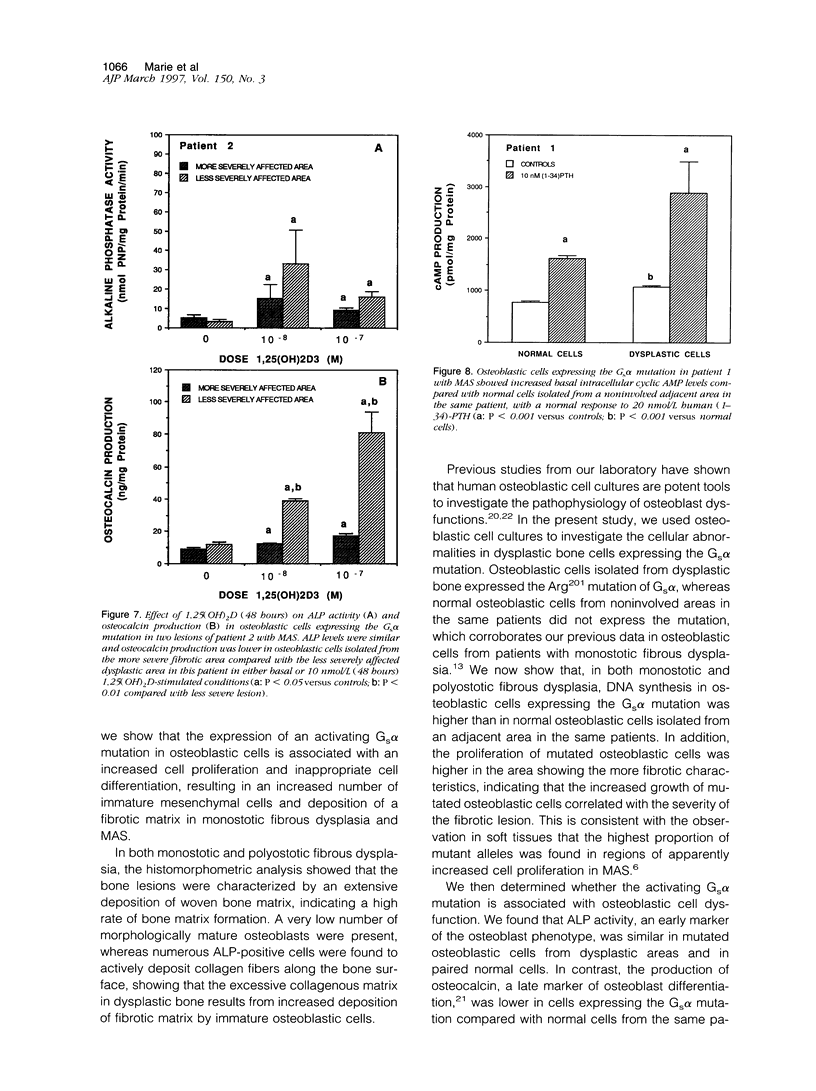
We studied the osteoblastic abnormalities resulting from activating mutation of the Gs alpha gene in two patients with McCune-Albright syndrome and one patient with monostotic fibrous dysplasia. Histomorphometric analysis of dysplastic lesions showed a low number of differentiated osteoblasts along the bone surface and numerous immature alkaline phosphatase-positive mesenchymal cells actively depositing a woven bone matrix. Osteoblastic cells isolated from dysplastic bone lesions expressed a missense mutation in the Gs alpha gene in position 201 and showed increased intracellular basal cyclic adenosine 3',5'-monophosphate levels compared with normal cells isolated from a noninvolved area in the same patient. Cell proliferation evaluated by DNA synthesis was two-fold to threefold greater in osteoblastic cells expressing the mutation compared with normal cells from the same patient and was greater in cells isolated from more severe than less severe fibrotic lesions. In contrast, the synthesis of osteocalcin, a marker of mature osteoblasts, was lower in osteoblastic cells expressing the Gs alpha mutation compared with normal cells from the same patient and was lower in cells isolated from severe compared with less severe fibrotic lesions, indicating that the increased growth in mutated osteoblastic cells was associated with reduced cell differentiation. The results show that activating mutation of Gs alpha in osteoblastic cells leads to constitutive activation of adenylate cyclase, increased cell proliferation, and inappropriate cell differentiation, resulting in overproduction of a disorganized fibrotic bone matrix in polyostotic and monostotic fibrous dysplasia.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Candeliere G. A., Glorieux F. H., Prud'homme J., St-Arnaud R. Increased expression of the c-fos proto-oncogene in bone from patients with fibrous dysplasia. N Engl J Med. 1995 Jun 8;332(23):1546–1551. doi: 10.1056/NEJM199506083322304. [DOI] [PubMed] [Google Scholar]
- Chanson P., Dib A., Visot A., Derome P. J. McCune-Albright syndrome and acromegaly: clinical studies and responses to treatment in five cases. Eur J Endocrinol. 1994 Sep;131(3):229–234. doi: 10.1530/eje.0.1310229. [DOI] [PubMed] [Google Scholar]
- Gaiddon C., Boutillier A. L., Monnier D., Mercken L., Loeffler J. P. Genomic effects of the putative oncogene G alpha s. Chronic transcriptional activation of the c-fos proto-oncogene in endocrine cells. J Biol Chem. 1994 Sep 9;269(36):22663–22671. [PubMed] [Google Scholar]
- Gaiddon C., Tian J., Loeffler J. P., Bancroft C. Constitutively active G(S) alpha-subunits stimulate Pit-1 promoter activity via a protein kinase A-mediated pathway acting through deoxyribonucleic acid binding sites both for Pit-1 and for adenosine 3',5'-monophosphate response element-binding protein. Endocrinology. 1996 Apr;137(4):1286–1291. doi: 10.1210/endo.137.4.8625901. [DOI] [PubMed] [Google Scholar]
- Grabias S. L., Campbell C. J. Fibrous dysplasia. Orthop Clin North Am. 1977 Oct;8(4):771–783. [PubMed] [Google Scholar]
- Grigoriadis A. E., Schellander K., Wang Z. Q., Wagner E. F. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol. 1993 Aug;122(3):685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hott M., Marie P. J. Glycol methacrylate as an embedding medium for bone. Stain Technol. 1987 Jan;62(1):51–57. doi: 10.3109/10520298709107965. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Harsh G., Lyons J., Davis R. L., McCormick F., Bourne H. R. Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J Clin Endocrinol Metab. 1990 Dec;71(6):1416–1420. doi: 10.1210/jcem-71-6-1416. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Ma Y. H., Landis C., Tchao N., Wang J., Rodd G., Hanahan D., Bourne H. R., Grodsky G. M. Constitutively active stimulatory G-protein alpha s in beta-cells of transgenic mice causes counterregulation of the increased adenosine 3',5'-monophosphate and insulin secretion. Endocrinology. 1994 Jan;134(1):42–47. doi: 10.1210/endo.134.1.7506212. [DOI] [PubMed] [Google Scholar]
- Machwate M., Jullienne A., Moukhtar M., Lomri A., Marie P. J. c-fos protooncogene is involved in the mitogenic effect of transforming growth factor-beta in osteoblastic cells. Mol Endocrinol. 1995 Feb;9(2):187–198. doi: 10.1210/mend.9.2.7776969. [DOI] [PubMed] [Google Scholar]
- Machwate M., Jullienne A., Moukhtar M., Marie P. J. Temporal variation of c-Fos proto-oncogene expression during osteoblast differentiation and osteogenesis in developing rat bone. J Cell Biochem. 1995 Jan;57(1):62–70. doi: 10.1002/jcb.240570108. [DOI] [PubMed] [Google Scholar]
- Malchoff C. D., Reardon G., MacGillivray D. C., Yamase H., Rogol A. D., Malchoff D. M. An unusual presentation of McCune-Albright syndrome confirmed by an activating mutation of the Gs alpha-subunit from a bone lesion. J Clin Endocrinol Metab. 1994 Mar;78(3):803–806. doi: 10.1210/jcem.78.3.8126161. [DOI] [PubMed] [Google Scholar]
- Marie P. J. Human osteoblastic cells: a potential tool to assess the etiology of pathologic bone formation. J Bone Miner Res. 1994 Dec;9(12):1847–1850. doi: 10.1002/jbmr.5650091202. [DOI] [PubMed] [Google Scholar]
- Marie P. J., Lomri A., Sabbagh A., Basle M. Culture and behavior of osteoblastic cells isolated from normal trabecular bone surfaces. In Vitro Cell Dev Biol. 1989 Apr;25(4):373–380. doi: 10.1007/BF02624601. [DOI] [PubMed] [Google Scholar]
- Marie P. J., de Vernejoul M. C., Connes D., Hott M. Decreased DNA synthesis by cultured osteoblastic cells in eugonadal osteoporotic men with defective bone formation. J Clin Invest. 1991 Oct;88(4):1167–1172. doi: 10.1172/JCI115418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters S. B., Miller R. T., Chi M. H., Chang F. H., Beiderman B., Lopez N. G., Bourne H. R. Mutations in the GTP-binding site of GS alpha alter stimulation of adenylyl cyclase. J Biol Chem. 1989 Sep 15;264(26):15467–15474. [PubMed] [Google Scholar]
- McCarthy T. L., Thomas M. J., Centrella M., Rotwein P. Regulation of insulin-like growth factor I transcription by cyclic adenosine 3',5'-monophosphate (cAMP) in fetal rat bone cells through an element within exon 1: protein kinase A-dependent control without a consensus AMP response element. Endocrinology. 1995 Sep;136(9):3901–3908. doi: 10.1210/endo.136.9.7649098. [DOI] [PubMed] [Google Scholar]
- Meissner J. D., Brown G. A., Mueller W. H., Scheibe R. J. Retinoic acid-mediated decrease of G (alpha S) protein expression: involvement of G (alpha S) in the differentiation of HL-60 myeloid cells. Exp Cell Res. 1996 May 25;225(1):112–121. doi: 10.1006/excr.1996.0162. [DOI] [PubMed] [Google Scholar]
- Modrowski D., Godet D., Marie P. J. Involvement of interleukin 1 and tumour necrosis factor alpha as endogenous growth factors in human osteoblastic cells. Cytokine. 1995 Oct;7(7):720–726. doi: 10.1006/cyto.1995.0085. [DOI] [PubMed] [Google Scholar]
- Shenker A., Chanson P., Weinstein L. S., Chi P., Spiegel A. M., Lomri A., Marie P. J. Osteoblastic cells derived from isolated lesions of fibrous dysplasia contain activating somatic mutations of the Gs alpha gene. Hum Mol Genet. 1995 Sep;4(9):1675–1676. doi: 10.1093/hmg/4.9.1675. [DOI] [PubMed] [Google Scholar]
- Shenker A., Weinstein L. S., Sweet D. E., Spiegel A. M. An activating Gs alpha mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J Clin Endocrinol Metab. 1994 Sep;79(3):750–755. doi: 10.1210/jcem.79.3.8077356. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Shenker A., Weinstein L. S. Receptor-effector coupling by G proteins: implications for normal and abnormal signal transduction. Endocr Rev. 1992 Aug;13(3):536–565. doi: 10.1210/edrv-13-3-536. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Weinstein L. S., Shenker A. Abnormalities in G protein-coupled signal transduction pathways in human disease. J Clin Invest. 1993 Sep;92(3):1119–1125. doi: 10.1172/JCI116680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar L., Spada A., Giannattasio G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987 Dec 10;330(6148):566–568. doi: 10.1038/330566a0. [DOI] [PubMed] [Google Scholar]
- Weinstein L. S., Shenker A., Gejman P. V., Merino M. J., Friedman E., Spiegel A. M. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991 Dec 12;325(24):1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ozono K., Kasayama S., Yoh K., Hiroshima K., Takagi M., Matsumoto S., Michigami T., Yamaoka K., Kishimoto T. Increased IL-6-production by cells isolated from the fibrous bone dysplasia tissues in patients with McCune-Albright syndrome. J Clin Invest. 1996 Jul 1;98(1):30–35. doi: 10.1172/JCI118773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Masters S. B., Bourne H. R. Increased mitogenic responsiveness of Swiss 3T3 cells expressing constitutively active Gs alpha. Biochem Biophys Res Commun. 1990 May 16;168(3):1184–1193. doi: 10.1016/0006-291x(90)91154-k. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., Sassone-Corsi P. Hormonal control of gene expression: multiplicity and versatility of cyclic adenosine 3',5'-monophosphate-responsive nuclear regulators. Mol Endocrinol. 1993 Feb;7(2):145–153. doi: 10.1210/mend.7.2.8385737. [DOI] [PubMed] [Google Scholar]