Abstract
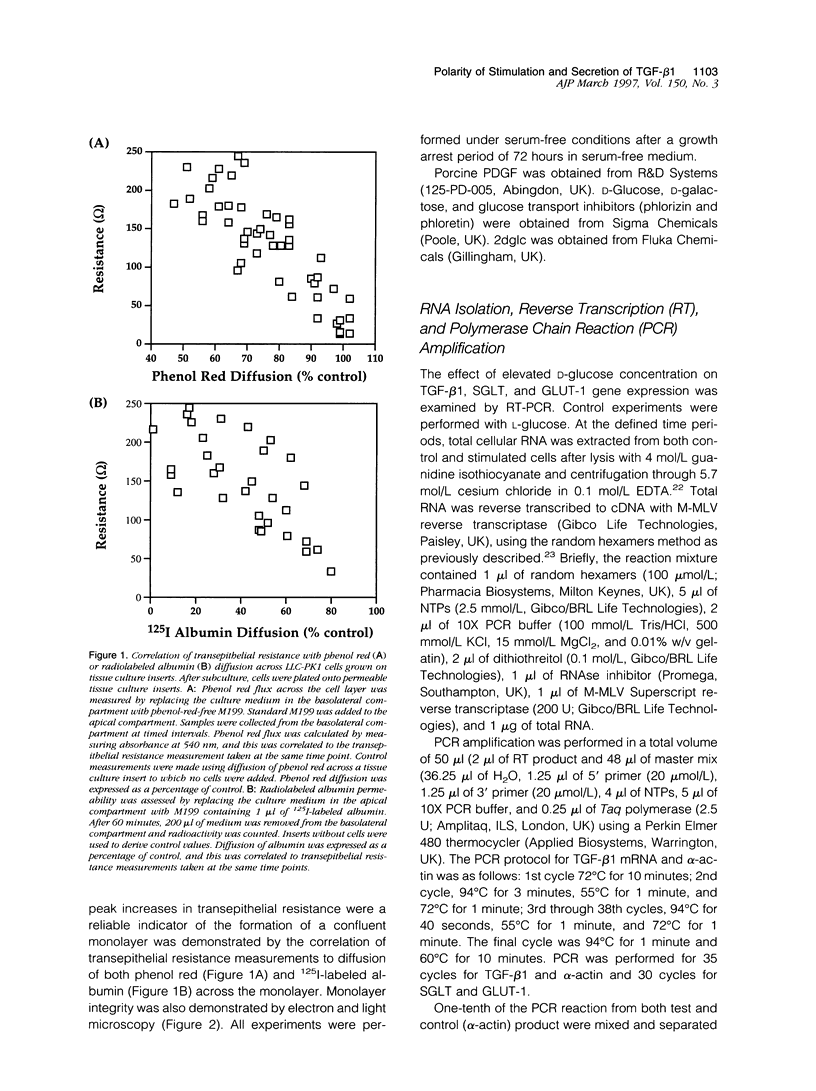
Proximal tubular epithelial cells are the most abundant cells in the renal cortex, and recent studies suggest that they may play an important role in initiating pathological changes in renal disease. Transforming growth factor (TGF)-beta 1 has been implicated as a major factor controlling the development and progression of renal fibrosis in numerous diseases, including diabetic nephropathy. We have recently demonstrated that human proximal tubular epithelial cells synthesize and secrete TGF-beta 1 after the sequential addition of both 25 mmol/L D-glucose and platelet-derived growth factor (PDGF). The present study examines the control of this synthesis and in particular the polar requirements of the stimulation and the direction of release of the protein. A proximal tubular cell line (LLC-PK1) was cultured on porous tissue culture inserts. Confluent cells were exposed to 25 mmol/L D-glucose on either their apical or basolateral aspect. TGF-beta 1 mRNA induction (reverse transcriptase polymerase chain reaction) occurred only after basolateral exposure. Similarly, TGF-beta 1 synthesis and secretion was induced only by the subsequent addition of PDGF to the basolateral aspect of the cells. In contrast, TGF-beta 1 protein secretion was detected equally in the apical and basolateral compartments. This effect was maximal after 12-hour PDGF stimulation and represented a threefold increase over controls for TGF-beta 1 in both the apical and basolateral compartments (n = 3, P < 0.05 versus control). The glucose transporter inhibitors phlorizin and phloretin were used to investigate the role of specific D-glucose transport proteins. Application of either basolateral phlorizin or phloretin at the time of addition of 25 mmol/L D-glucose to the same compartment inhibited TGF-beta 1 synthesis in response to PDGF. Maximal inhibition was achieved at 0.5 mmol/L of either inhibitor (phlorizin percent inhibition of apical TGF-beta 1, 75%, P = 0.015, and of basolateral TGF-beta 1, 78%, P = 0.015; phloretin percent inhibition of apical TGF-beta 1, 68%, P = 0.03, and of basolateral TGF-beta 1, 79%, P = 0.001, n = 5, P versus control). No inhibition was seen with apical application of either inhibitor. These data demonstrate that the priming of proximal tubular cells for TGF-beta 1 synthesis occurs only after basolateral exposure of the cells to 25 mmol/L D-glucose. This mechanism is dependent on the activity of the basolateral D-glucose transporter GLUT-1. In another series of experiments, TGF-beta 1 synthesis in response to the addition of basolateral PDGF was also induced after basolateral pretreatment with D-galactose but not 2-deoxy-D-glucose. This priming effect demonstrates the dependence of this response on glucose metabolism by the cells, not simply the activity of the GLUT-1 transporter, as both 2-deoxy-D-glucose and D-galactose are transported by GLUT-1, although only the latter is metabolized. The extrapolation of these results to diabetic nephropathy would suggest that it is changes in the interstitial concentration of glucose rather than the urinary glucose level that likely modulate the synthesis of the profibrotic cytokine TGF-beta 1 and thereby influence the progression of interstitial fibrosis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank N., Aynedjian H. S. Progressive increases in luminal glucose stimulate proximal sodium absorption in normal and diabetic rats. J Clin Invest. 1990 Jul;86(1):309–316. doi: 10.1172/JCI114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer-Mears A., Ku L., Cohen M. P. Glomerular polyol accumulation in diabetes and its prevention by oral sorbinil. Diabetes. 1984 Jun;33(6):604–607. doi: 10.2337/diab.33.6.604. [DOI] [PubMed] [Google Scholar]
- Bleyer A. J., Fumo P., Snipes E. R., Goldfarb S., Simmons D. A., Ziyadeh F. N. Polyol pathway mediates high glucose-induced collagen synthesis in proximal tubule. Kidney Int. 1994 Mar;45(3):659–666. doi: 10.1038/ki.1994.88. [DOI] [PubMed] [Google Scholar]
- Bohle A., Wehrmann M., Bogenschütz O., Batz C., Müller C. A., Müller G. A. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991 Mar;187(2-3):251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990 Feb;37(2):689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Davidson C. M., DeRubertis F. R. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990 Jun;39(6):667–674. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- Guillausseau P. J., Dupuy E., Bryckaert M. C., Timsit J., Chanson P., Tobelem G., Caen J. P., Lubetzki J. Platelet-derived growth factor (PDGF) in type 1 diabetes mellitus. Eur J Clin Invest. 1989 Apr;19(2):172–175. doi: 10.1111/j.1365-2362.1989.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Handberg A., Vaag A., Damsbo P., Beck-Nielsen H., Vinten J. Expression of insulin regulatable glucose transporters in skeletal muscle from type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990 Oct;33(10):625–627. doi: 10.1007/BF00400207. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Jovov B., Wills N. K., Lewis S. A. A spectroscopic method for assessing confluence of epithelial cell cultures. Am J Physiol. 1991 Dec;261(6 Pt 1):C1196–C1203. doi: 10.1152/ajpcell.1991.261.6.C1196. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- Lane P. H., Steffes M. W., Fioretto P., Mauer S. M. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int. 1993 Mar;43(3):661–667. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- Mizutani M., Okuda Y., Yamaoka T., Tsukahara K., Isaka M., Bannai C., Yamashita K. High glucose and hyperosmolarity increase platelet-derived growth factor mRNA levels in cultured human vascular endothelial cells. Biochem Biophys Res Commun. 1992 Sep 16;187(2):664–669. doi: 10.1016/0006-291x(92)91246-m. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Fukui M., Ebihara I., Osada S., Nagaoka I., Tomino Y., Koide H. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes. 1993 Mar;42(3):450–456. doi: 10.2337/diab.42.3.450. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Miller D., Ruoslahti E., Border W. A. Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-beta 1. Kidney Int. 1992 May;41(5):1213–1221. doi: 10.1038/ki.1992.183. [DOI] [PubMed] [Google Scholar]
- Neubauer A., Neubauer B., Liu E. Polymerase chain reaction based assay to detect allelic loss in human DNA: loss of beta-interferon gene in chronic myelogenous leukemia. Nucleic Acids Res. 1990 Feb 25;18(4):993–998. doi: 10.1093/nar/18.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Yoon K., Prince C. W., Butler W. T., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J Biol Chem. 1988 Sep 25;263(27):13916–13921. [PubMed] [Google Scholar]
- O'Bryan J. P., Frye R. A., Cogswell P. C., Neubauer A., Kitch B., Prokop C., Espinosa R., 3rd, Le Beau M. M., Earp H. S., Liu E. T. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991 Oct;11(10):5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Isselbacher K. J., Rhoads D. B. Regulation of glucose transporters in LLC-PK1 cells: effects of D-glucose and monosaccharides. Mol Cell Biol. 1990 Dec;10(12):6491–6499. doi: 10.1128/mcb.10.12.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. O., Steadman R., Donovan K. D., Williams J. D. A new antibody capture enzyme linked immunoassay specific for transforming growth factor beta 1. Int J Biochem Cell Biol. 1995 Feb;27(2):207–213. doi: 10.1016/1357-2725(94)00077-o. [DOI] [PubMed] [Google Scholar]
- Phillips A. O., Steadman R., Topley N., Williams J. D. Elevated D-glucose concentrations modulate TGF-beta 1 synthesis by human cultured renal proximal tubular cells. The permissive role of platelet-derived growth factor. Am J Pathol. 1995 Aug;147(2):362–374. [PMC free article] [PubMed] [Google Scholar]
- Shankland S. J., Scholey J. W., Ly H., Thai K. Expression of transforming growth factor-beta 1 during diabetic renal hypertrophy. Kidney Int. 1994 Aug;46(2):430–442. doi: 10.1038/ki.1994.291. [DOI] [PubMed] [Google Scholar]
- Sharma K., Jin Y., Guo J., Ziyadeh F. N. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996 Apr;45(4):522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- Silverman M. Glucose transport in the kidney. Biochim Biophys Acta. 1976 Dec 14;457(3-4):303–351. doi: 10.1016/0304-4157(76)90003-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakamura T., Noble N. A., Ruoslahti E., Border W. A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. A., Johnson R. J., Alpers C. E., Eng E., Gordon K., Floege J., Couser W. G., Seidel K. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995 Mar;47(3):935–944. doi: 10.1038/ki.1995.139. [DOI] [PubMed] [Google Scholar]