Abstract
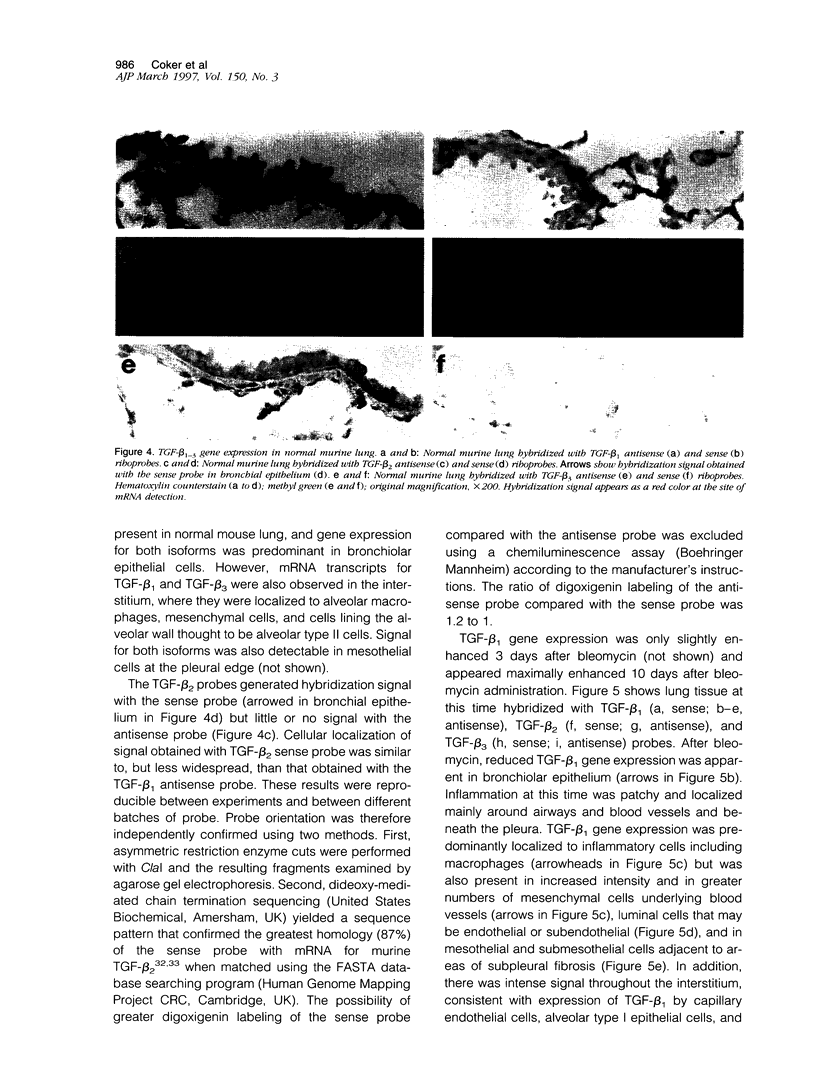
Transforming growth factor (TGF)-beta 1 may potentiate wound healing and fibrosis by stimulating fibroblast collagen deposition. TGF-beta 1 is implicated in the pathogenesis of pulmonary fibrosis, but the role of TGF-beta 2 and TGF-beta 3 remains unclear. We examined their effects on lung fibroblast procollagen metabolism in vitro and localized their gene expression during bleomycin-induced lung fibrosis using in situ hybridization with digoxigenin-labeled riboprobes. All three isoforms stimulated fibroblast procollagen production. TGF-beta 3 was the most potent and also reduced procollagen degradation. In normal mouse lung, TGF-beta 1 and TGF-beta 3 mRNA transcripts were abundant in bronchiolar epithelium. After bleomycin, TGF-beta 1 gene expression was maximally enhanced at 10 days, with the signal being predominant in macrophages. Signal was also enhanced in mesenchymal, pulmonary endothelial, and mesothelial cells. After 35 days, the pattern of TGF-beta 1 gene expression returned to that of control lung. TGF-beta 3 gene expression remained unchanged throughout compared with controls. TGF-beta 2 mRNA was not detected with the antisense probe, but signal obtained with the sense probe suggests the presence of a naturally occurring antisense. This study demonstrates that TGF-beta 1, -beta 2, and -beta 3 all exert profibrotic effects in vitro. However, TGF-beta isoform gene expression is differentially controlled during experimental pulmonary fibrosis with TGF-beta 1 the predominant isoform expressed during pathogenesis.
Full text
PDF







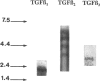
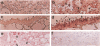
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bakowska J., Bowden D. H. Mesothelial cell proliferation: a nonspecific response to lung injury associated with fibrosis. Am J Respir Cell Mol Biol. 1994 Mar;10(3):253–258. doi: 10.1165/ajrcmb.10.3.7509611. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Bleomycin-induced injury and metaplasia of alveolar type 2 cells. Relationship of cellular responses to drug presence in the lung. Am J Pathol. 1979 Aug;96(2):531–544. [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C. M., Barth R. K. PCR analysis of cytokine induction profiles associated with mouse strain variation in susceptibility to pulmonary fibrosis. Reg Immunol. 1993 May-Aug;5(3-4):207–217. [PubMed] [Google Scholar]
- Bermudez E., Everitt J., Walker C. Expression of growth factor and growth factor receptor RNA in rat pleural mesothelial cells in culture. Exp Cell Res. 1990 Sep;190(1):91–98. doi: 10.1016/0014-4827(90)90148-4. [DOI] [PubMed] [Google Scholar]
- Breen E., Shull S., Burne S., Absher M., Kelley J., Phan S., Cutroneo K. R. Bleomycin regulation of transforming growth factor-beta mRNA in rat lung fibroblasts. Am J Respir Cell Mol Biol. 1992 Feb;6(2):146–152. doi: 10.1165/ajrcmb/6.2.146. [DOI] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa J. S., McAnulty R. J., Laurent G. J. Application of high-pressure liquid chromatography to studies of collagen production by isolated cells in culture. Anal Biochem. 1990 May 1;186(2):257–263. doi: 10.1016/0003-2697(90)90076-l. [DOI] [PubMed] [Google Scholar]
- Chambers R. C., McAnulty R. J., Shock A., Campa J. S., Newman Taylor A. J., Laurent G. J. Cadmium selectively inhibits fibroblast procollagen production and proliferation. Am J Physiol. 1994 Sep;267(3 Pt 1):L300–L308. doi: 10.1152/ajplung.1994.267.3.L300. [DOI] [PubMed] [Google Scholar]
- Corrin B., Butcher D., McAnulty B. J., Dubois R. M., Black C. M., Laurent G. J., Harrison N. K. Immunohistochemical localization of transforming growth factor-beta 1 in the lungs of patients with systemic sclerosis, cryptogenic fibrosing alveolitis and other lung disorders. Histopathology. 1994 Feb;24(2):145–150. doi: 10.1111/j.1365-2559.1994.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Davila R. M., Crouch E. C. Role of mesothelial and submesothelial stromal cells in matrix remodeling following pleural injury. Am J Pathol. 1993 Feb;142(2):547–555. [PMC free article] [PubMed] [Google Scholar]
- Denis M. Neutralization of transforming growth factor-beta 1 in a mouse model of immune-induced lung fibrosis. Immunology. 1994 Aug;82(4):584–590. [PMC free article] [PubMed] [Google Scholar]
- Franzén L., Dahlquist C. The effect of transforming growth factor-beta on fibroblast cell proliferation in intact connective tissue in vitro. In Vitro Cell Dev Biol Anim. 1994 Jul;30A(7):460–463. doi: 10.1007/BF02631314. [DOI] [PubMed] [Google Scholar]
- Gabrielson E. W., Gerwin B. I., Harris C. C., Roberts A. B., Sporn M. B., Lechner J. F. Stimulation of DNA synthesis in cultured primary human mesothelial cells by specific growth factors. FASEB J. 1988 Aug;2(11):2717–2721. doi: 10.1096/fasebj.2.11.3260881. [DOI] [PubMed] [Google Scholar]
- Gatherer D., Ten Dijke P., Baird D. T., Akhurst R. J. Expression of TGF-beta isoforms during first trimester human embryogenesis. Development. 1990 Oct;110(2):445–460. doi: 10.1242/dev.110.2.445. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Lechner J. F., Reddel R. R., Roberts A. B., Robbins K. C., Gabrielson E. W., Harris C. C. Comparison of production of transforming growth factor-beta and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res. 1987 Dec 1;47(23):6180–6184. [PubMed] [Google Scholar]
- Giri S. N., Hyde D. M., Hollinger M. A. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993 Oct;48(10):959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt D. G., Lazo J. S. Alterations in pulmonary mRNA encoding procollagens, fibronectin and transforming growth factor-beta precede bleomycin-induced pulmonary fibrosis in mice. J Pharmacol Exp Ther. 1988 Aug;246(2):765–771. [PubMed] [Google Scholar]
- Kelley J., Fabisiak J. P., Hawes K., Absher M. Cytokine signaling in lung: transforming growth factor-beta secretion by lung fibroblasts. Am J Physiol. 1991 Feb;260(2 Pt 1):L123–L128. doi: 10.1152/ajplung.1991.260.2.L123. [DOI] [PubMed] [Google Scholar]
- Kelley J., Shull S., Walsh J. J., Cutroneo K. R., Absher M. Auto-induction of transforming growth factor-beta in human lung fibroblasts. Am J Respir Cell Mol Biol. 1993 Apr;8(4):417–424. doi: 10.1165/ajrcmb/8.4.417. [DOI] [PubMed] [Google Scholar]
- Khalil N., Bereznay O., Sporn M., Greenberg A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989 Sep 1;170(3):727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N., O'Connor R. N., Flanders K. C., Shing W., Whitman C. I. Regulation of type II alveolar epithelial cell proliferation by TGF-beta during bleomycin-induced lung injury in rats. Am J Physiol. 1994 Nov;267(5 Pt 1):L498–L507. doi: 10.1152/ajplung.1994.267.5.L498. [DOI] [PubMed] [Google Scholar]
- Khalil N., O'Connor R. N., Unruh H. W., Warren P. W., Flanders K. C., Kemp A., Bereznay O. H., Greenberg A. H. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991 Aug;5(2):155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- Khalil N., Whitman C., Zuo L., Danielpour D., Greenberg A. Regulation of alveolar macrophage transforming growth factor-beta secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest. 1993 Oct;92(4):1812–1818. doi: 10.1172/JCI116771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. J., McAnulty R. J., Corrin B., Cockerill P. Biochemical and histological changes in pulmonary fibrosis induced in rabbits with intratracheal bleomycin. Eur J Clin Invest. 1981 Dec;11(6):441–448. doi: 10.1111/j.1365-2362.1981.tb02011.x. [DOI] [PubMed] [Google Scholar]
- Madisen L., Webb N. R., Rose T. M., Marquardt H., Ikeda T., Twardzik D., Seyedin S., Purchio A. F. Transforming growth factor-beta 2: cDNA cloning and sequence analysis. DNA. 1988 Jan-Feb;7(1):1–8. doi: 10.1089/dna.1988.7.1. [DOI] [PubMed] [Google Scholar]
- Masui T., Wakefield L. M., Lechner J. F., LaVeck M. A., Sporn M. B., Harris C. C. Type beta transforming growth factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2438–2442. doi: 10.1073/pnas.83.8.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty R. J., Campa J. S., Cambrey A. D., Laurent G. J. The effect of transforming growth factor beta on rates of procollagen synthesis and degradation in vitro. Biochim Biophys Acta. 1991 Jan 31;1091(2):231–235. doi: 10.1016/0167-4889(91)90066-7. [DOI] [PubMed] [Google Scholar]
- McAnulty R. J., Chambers R. C., Laurent G. J. Regulation of fibroblast procollagen production. Transforming growth factor-beta 1 induces prostaglandin E2 but not procollagen synthesis via a pertussis toxin-sensitive G-protein. Biochem J. 1995 Apr 1;307(Pt 1):63–68. doi: 10.1042/bj3070063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty R. J., Laurent G. J. Pathogenesis of lung fibrosis and potential new therapeutic strategies. Exp Nephrol. 1995 Mar-Apr;3(2):96–107. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Stein H., Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991 Dec;139(6):1221–1229. [PMC free article] [PubMed] [Google Scholar]
- Millan F. A., Denhez F., Kondaiah P., Akhurst R. J. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991 Jan;111(1):131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Müller G., Behrens J., Nussbaumer U., Böhlen P., Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M. W., Grimes S. R. Pleural mesothelial cell response to inflammation: tumor necrosis factor-induced mitogenesis and collagen synthesis. Am J Physiol. 1993 Oct;265(4 Pt 1):L382–L388. doi: 10.1152/ajplung.1993.265.4.L382. [DOI] [PubMed] [Google Scholar]
- Pelton R. W., Johnson M. D., Perkett E. A., Gold L. I., Moses H. L. Expression of transforming growth factor-beta 1, -beta 2, and -beta 3 mRNA and protein in the murine lung. Am J Respir Cell Mol Biol. 1991 Dec;5(6):522–530. doi: 10.1165/ajrcmb/5.6.522. [DOI] [PubMed] [Google Scholar]
- Perkett E. A., Pelton R. W., Meyrick B., Gold L. I., Miller D. A. Expression of transforming growth factor-beta mRNAs and proteins in pulmonary vascular remodeling in the sheep air embolization model of pulmonary hypertension. Am J Respir Cell Mol Biol. 1994 Jul;11(1):16–24. doi: 10.1165/ajrcmb.11.1.8018335. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Gharee-Kermani M., Wolber F., Ryan U. S. Bleomycin stimulates production of transforming growth factor-beta by rat pulmonary artery endothelial cells. Chest. 1991 Mar;99(3 Suppl):66S–66S. doi: 10.1378/chest.99.3_supplement.66s-a. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Kunkel S. L. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992 Jan-Mar;18(1):29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Wilkinson M. M. Inhibition of endogenous tissue alkaline phosphatase with the use of alkaline phosphatase conjugates in immunohistochemistry. J Histochem Cytochem. 1981 Aug;29(8):981–984. doi: 10.1177/29.8.7024402. [DOI] [PubMed] [Google Scholar]
- Potts J. D., Vincent E. B., Runyan R. B., Weeks D. L. Sense and antisense TGF beta 3 mRNA levels correlate with cardiac valve induction. Dev Dyn. 1992 Apr;193(4):340–345. doi: 10.1002/aja.1001930407. [DOI] [PubMed] [Google Scholar]
- Raghow B., Irish P., Kang A. H. Coordinate regulation of transforming growth factor beta gene expression and cell proliferation in hamster lungs undergoing bleomycin-induced pulmonary fibrosis. J Clin Invest. 1989 Dec;84(6):1836–1842. doi: 10.1172/JCI114369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana A., Saxena B., Noble N. A., Gold L. I., Marshall B. C. Increased expression of transforming growth factor beta isoforms (beta 1, beta 2, beta 3) in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1995 Jul;13(1):34–44. doi: 10.1165/ajrcmb.13.1.7541221. [DOI] [PubMed] [Google Scholar]
- Schmid P., Cox D., Bilbe G., Maier R., McMaster G. K. Differential expression of TGF beta 1, beta 2 and beta 3 genes during mouse embryogenesis. Development. 1991 Jan;111(1):117–130. doi: 10.1242/dev.111.1.117. [DOI] [PubMed] [Google Scholar]
- Schmid P., Cox D., Bilbe G., McMaster G., Morrison C., Stähelin H., Lüscher N., Seiler W. TGF-beta s and TGF-beta type II receptor in human epidermis: differential expression in acute and chronic skin wounds. J Pathol. 1993 Nov;171(3):191–197. doi: 10.1002/path.1711710307. [DOI] [PubMed] [Google Scholar]
- Shahzeidi S., Jeffery P. K., Laurent G. J., McAnulty R. J. Increased type I procollagen mRNA transcripts in the lungs of mice during the development of bleomycin-induced fibrosis. Eur Respir J. 1994 Nov;7(11):1938–1943. [PubMed] [Google Scholar]
- Shahzeidi S., Mulier B., de Crombrugghe B., Jeffery P. K., McAnulty R. J., Laurent G. J. Enhanced type III collagen gene expression during bleomycin induced lung fibrosis. Thorax. 1993 Jun;48(6):622–628. doi: 10.1136/thx.48.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsicker K., Flanders K. C., Cissel D. S., Lafyatis R., Sporn M. B. Transforming growth factor beta isoforms in the adult rat central and peripheral nervous system. Neuroscience. 1991;44(3):613–625. doi: 10.1016/0306-4522(91)90082-y. [DOI] [PubMed] [Google Scholar]
- Webb N. R., Madisen L., Rose T. M., Purchio A. F. Structural and sequence analysis of TGF-beta 2 cDNA clones predicts two different precursor proteins produced by alternative mRNA splicing. DNA. 1988 Sep;7(7):493–497. doi: 10.1089/dna.1.1988.7.493. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y., Friess H., Büchler M., Beger H. G., Gold L. I., Korc M. Synthesis and expression of transforming growth factor beta-1, beta-2, and beta-3 in the endocrine and exocrine pancreas. Diabetes. 1993 May;42(5):746–756. doi: 10.2337/diab.42.5.746. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Iwata K. K., Goddard C., Pieler C., Canalis E., McCarthy T. L., Centrella M. Recombinant transforming growth factor type beta 3: biological activities and receptor-binding properties in isolated bone cells. Mol Cell Biol. 1990 Sep;10(9):4473–4479. doi: 10.1128/mcb.10.9.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]