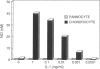Abstract
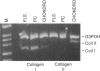
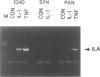
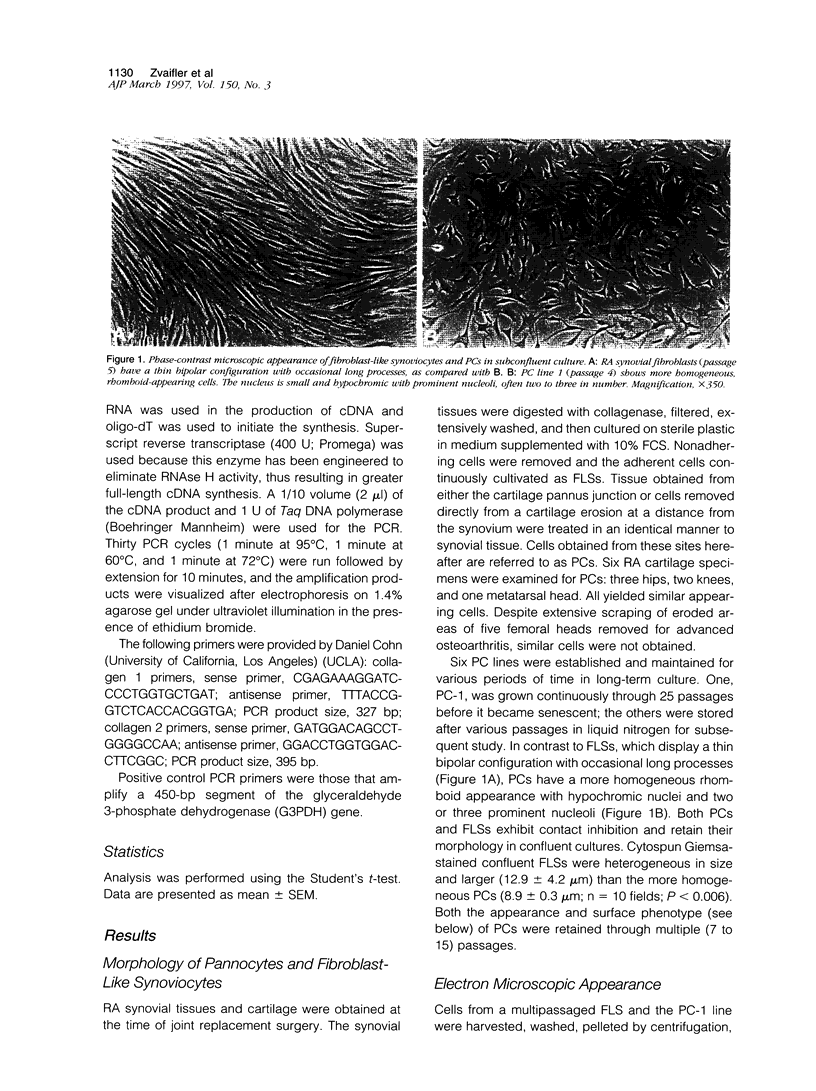
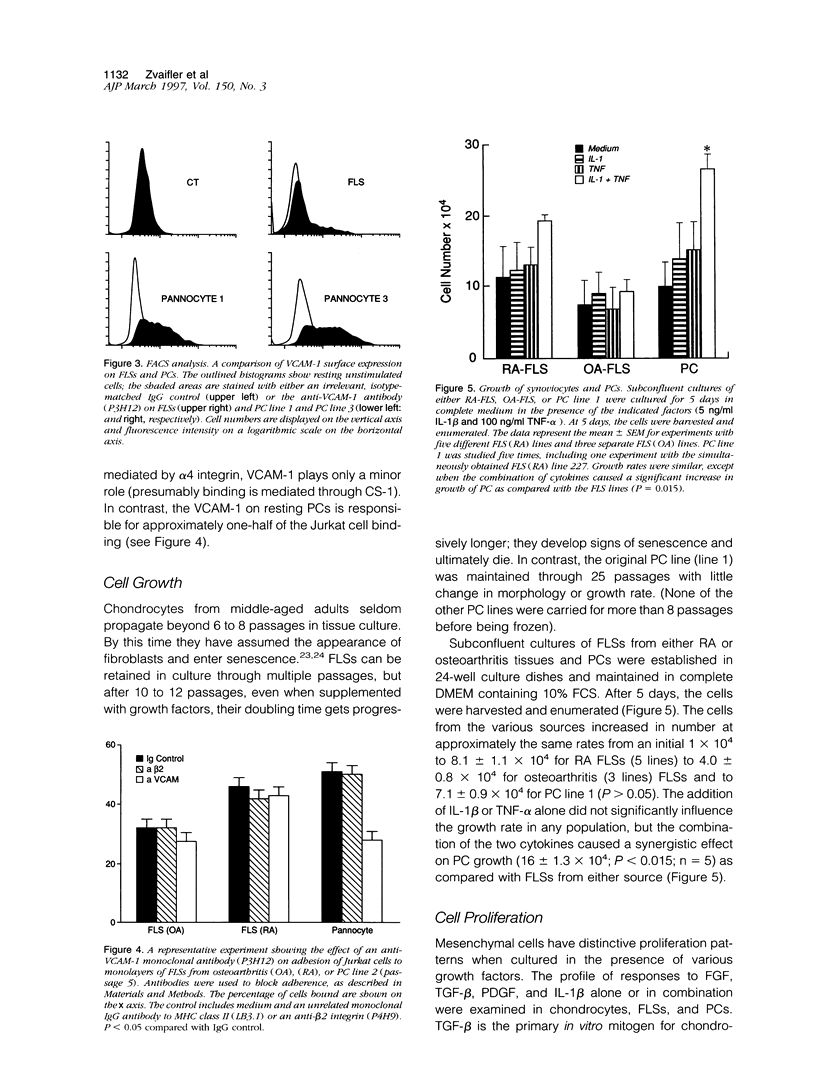
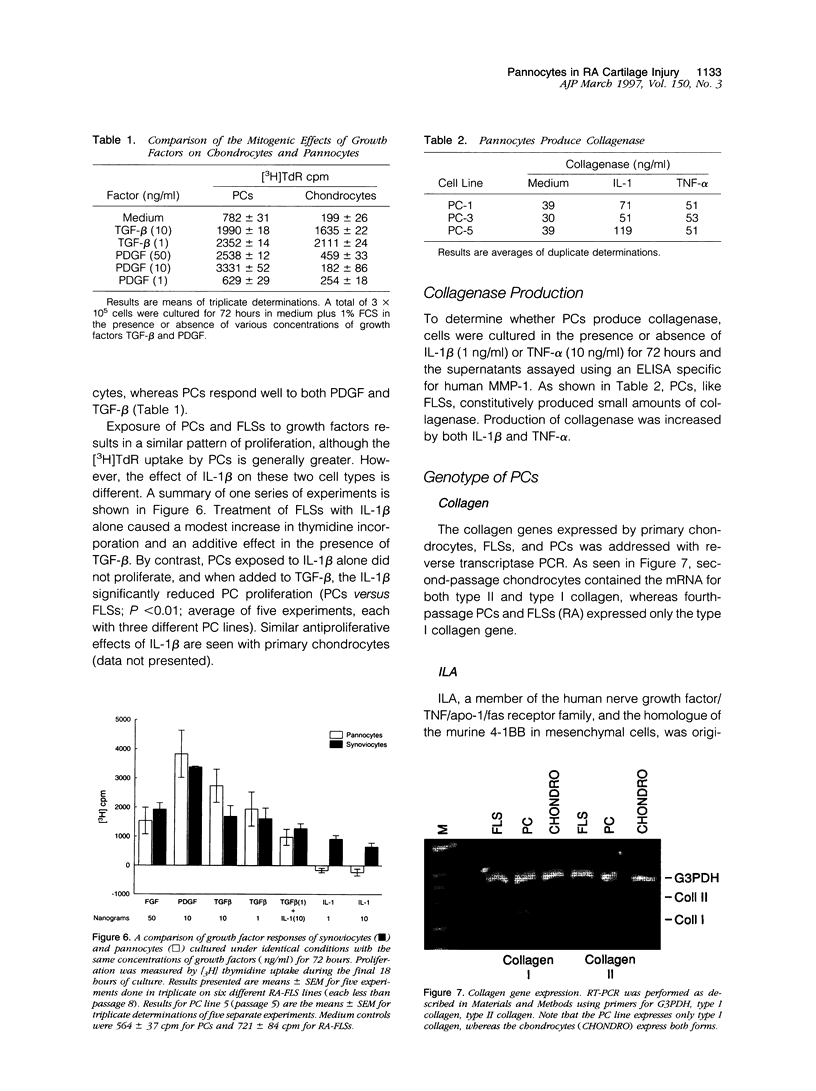
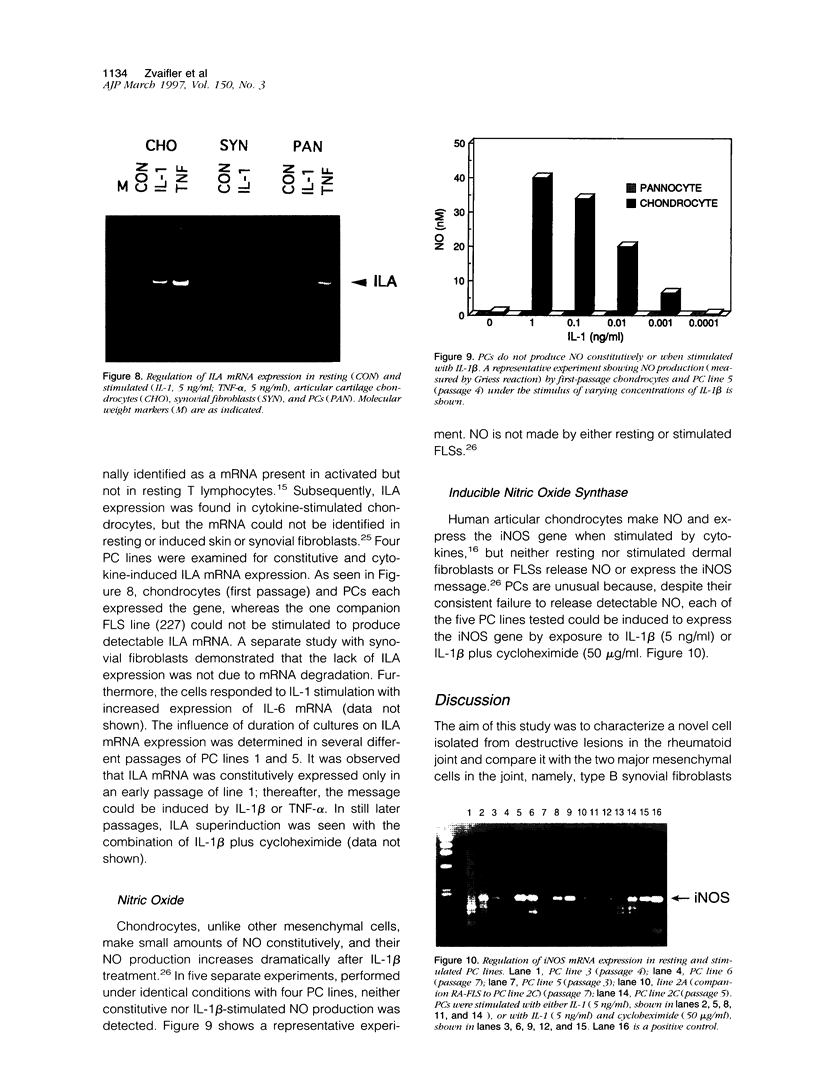
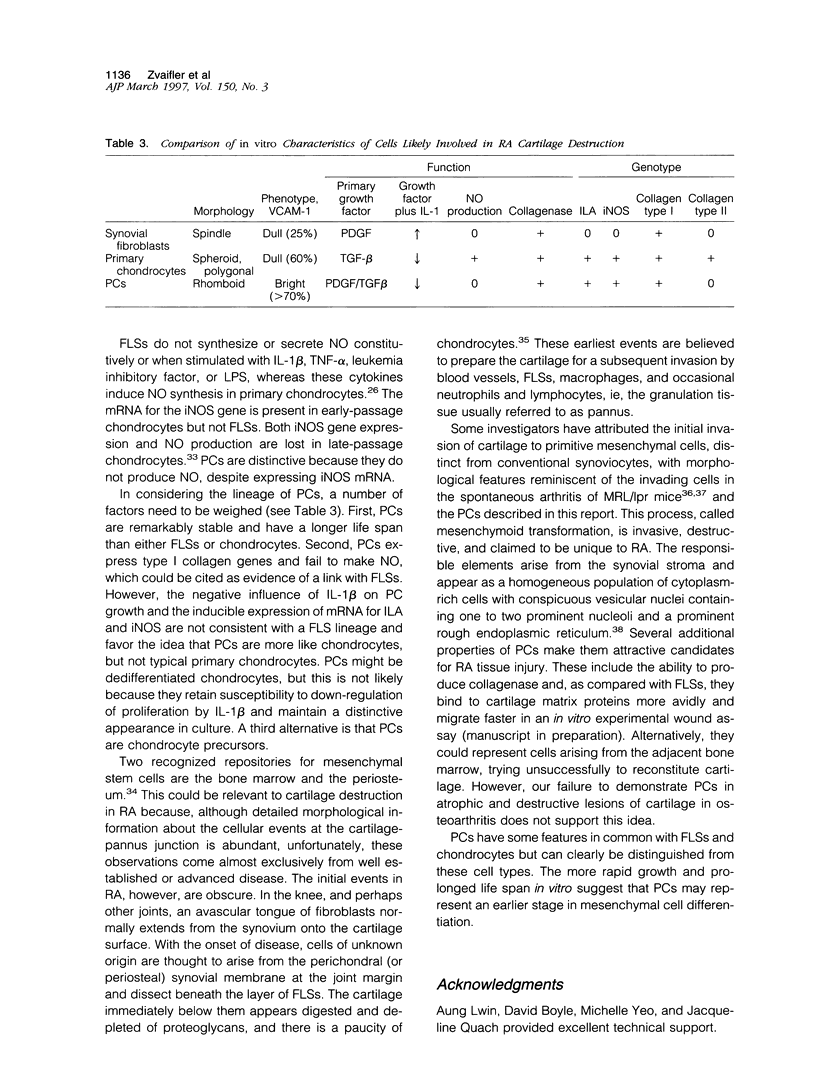
A distinctive cell was identified from sites of rheumatoid arthritis cartilage injury. Similar cells are not found in lesions of osteoarthritis cartilage. We have designated them as pannocytes (PCs). Their rhomboid morphology differs from the bipolar shape of fibroblast-like synoviocytes or the spherical configuration of primary human articular chondrocytes. Chondrocytes are short-lived, whereas the original PC line grew for 25 passages before becoming senescent. Features in common with cultured primary chondrocytes include maximal proliferation in response to transforming growth factor-beta a catabolic response to interleukin-1 beta, collagenase production, and mRNA for the induced lymphocyte antigen and inducible nitric oxide synthase. Despite the presence of the inducible nitric oxide synthase message, PCs do not produce NO either constitutively or when cytokine stimulated. Each of the mesenchymal cells, fibroblast-like synoviocytes, primary chondrocytes, and PCs have the gene for type I collagen, but the type II collagen gene is detected only in primary chondrocytes. PCs can be distinguished from fibroblast-like synoviocytes and primary chondrocytes by their morphology, bright VCAM-1 staining, and growth response to cytokines and growth factors. Their prolonged life span in vitro suggests that PCs might represent an earlier stage of mesenchymal cell differentiation, and they could have a heretofore unrecognized role in rheumatoid arthritis joint destruction.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Allard S. A., Bayliss M. T., Maini R. N. The synovium-cartilage junction of the normal human knee. Implications for joint destruction and repair. Arthritis Rheum. 1990 Aug;33(8):1170–1179. doi: 10.1002/art.1780330818. [DOI] [PubMed] [Google Scholar]
- Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Cytokines in chronic inflammatory arthritis. IV. Granulocyte/macrophage colony-stimulating factor-mediated induction of class II MHC antigen on human monocytes: a possible role in rheumatoid arthritis. J Exp Med. 1989 Sep 1;170(3):865–875. doi: 10.1084/jem.170.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990 Dec;86(6):1790–1798. doi: 10.1172/JCI114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLAND P., NOVIKOFF A. B., HAMERMAN D. Electron microscopy of the human synovial membrane. J Cell Biol. 1962 Aug;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Geng Y., Lotz M. Differentiation-dependent effects of IL-1 and TGF-beta on human articular chondrocyte proliferation are related to inducible nitric oxide synthase expression. J Immunol. 1995 Apr 15;154(8):4018–4026. [PubMed] [Google Scholar]
- Burgeson R. E. New collagens, new concepts. Annu Rev Cell Biol. 1988;4:551–577. doi: 10.1146/annurev.cb.04.110188.003003. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Caplan A. I. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Evans C. H., Georgescu H. I. Observations on the senescence of cells derived from articular cartilage. Mech Ageing Dev. 1983 Jun;22(2):179–191. doi: 10.1016/0047-6374(83)90111-2. [DOI] [PubMed] [Google Scholar]
- Fassbender H. G. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983 Mar;3(2):141–155. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M., Littman B. H. Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991 Sep;34(9):1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Tsai V., Zvaifler N. J. Cellular immunity in the joints of patients with rheumatoid arthritis and other forms of chronic synovitis. Rheum Dis Clin North Am. 1987 Aug;13(2):191–213. [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Sublet A., Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. J Cell Physiol. 1994 Mar;158(3):476–484. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Elices M. J., Parker C., Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990 Apr;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Functional heterogeneity of human rheumatoid synovial tissue macrophages. J Rheumatol. 1988 Jul;15(7):1058–1063. [PubMed] [Google Scholar]
- Krane S. M., Conca W., Stephenson M. L., Amento E. P., Goldring M. B. Mechanisms of matrix degradation in rheumatoid arthritis. Ann N Y Acad Sci. 1990;580:340–354. doi: 10.1111/j.1749-6632.1990.tb17943.x. [DOI] [PubMed] [Google Scholar]
- Maier R., Bilbe G., Rediske J., Lotz M. Inducible nitric oxide synthase from human articular chondrocytes: cDNA cloning and analysis of mRNA expression. Biochim Biophys Acta. 1994 Sep 21;1208(1):145–150. doi: 10.1016/0167-4838(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Morales-Ducret J., Wayner E., Elices M. J., Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Alpha 4/beta 1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992 Aug 15;149(4):1424–1431. [PubMed] [Google Scholar]
- O'Sullivan F. X., Fassbender H. G., Gay S., Koopman W. J. Etiopathogenesis of the rheumatoid arthritis-like disease in MRL/l mice. I. The histomorphologic basis of joint destruction. Arthritis Rheum. 1985 May;28(5):529–536. doi: 10.1002/art.1780280511. [DOI] [PubMed] [Google Scholar]
- Rediske J. J., Koehne C. F., Zhang B., Lotz M. The inducible production of nitric oxide by articular cell types. Osteoarthritis Cartilage. 1994 Sep;2(3):199–206. doi: 10.1016/s1063-4584(05)80069-x. [DOI] [PubMed] [Google Scholar]
- Remmers E. F., Sano H., Wilder R. L. Platelet-derived growth factors and heparin-binding (fibroblast) growth factors in the synovial tissue pathology of rheumatoid arthritis. Semin Arthritis Rheum. 1991 Dec;21(3):191–199. doi: 10.1016/0049-0172(91)90009-o. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Schürch W., Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990 Aug;63(2):144–161. [PubMed] [Google Scholar]
- Schwarz H., Tuckwell J., Lotz M. A receptor induced by lymphocyte activation (ILA): a new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene. 1993 Dec 8;134(2):295–298. doi: 10.1016/0378-1119(93)90110-o. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Cheung N. T., Dawes P. T. Increased serum proMMP-3 in inflammatory arthritides: a potential indicator of synovial inflammatory monokine activity. Ann Rheum Dis. 1994 Nov;53(11):768–772. doi: 10.1136/ard.53.11.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Carson D. A., Tavassoli M., Slovin S. F., Speers W. C., Jensen F. B., Vaughan J. H. Evidence for the presence of receptors for C3 and IgG Fc on human synovial cells. Arthritis Rheum. 1980 Jan;23(1):1–9. doi: 10.1002/art.1780230102. [DOI] [PubMed] [Google Scholar]
- Vuorio E. Rheumatoid disease in cultured human synovial cells. A biochemical study on glycosaminoglycans, proteins and plasma membranes of synovial fibroblasts in culture. Scand J Clin Lab Invest Suppl. 1977;(149):1–72. [PubMed] [Google Scholar]
- Zvaifler N. J., Firestein G. S. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994 Jun;37(6):783–789. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]