Abstract
Signaling by the metabotropic glutamate receptor 1α (mGluR1α) can lead to the accumulation of inositol 1,4,5-trisphosphate (InsP3) and cAMP and to the modulation of K+ and Ca2+ channel opening. At present, very little is known about how these different actions are integrated and eventually turned off. Unraveling the molecular mechanisms underlying these functions is crucial for understanding mGluR-mediated regulation of synaptic transmission. It has been shown that receptor-induced activation of the InsP3 pathway is subject to feedback inhibition mediated by protein kinase C (PKC). In this study, we provide evidence for a differential regulation by PKC and protein kinase A of two distinct mGluR1α-dependent signaling pathways. PKC activation selectively inhibits agonist-dependent stimulation of the InsP3 pathway but does not affect receptor signaling via cAMP. In contrast, protein kinase A potentiates agonist-independent signaling of the receptor via InsP3. Furthermore, we demonstrate that the selectivity of PKC action on receptor signaling rests on phosphorylation of a threonine residue located in the G protein-interacting domain of the receptor. Modification at Thr695 selectively disrupts mGluR1α–Gq/11 interaction without affecting signaling through Gs. Together, these data provide insight on the mechanisms by which selective down-regulation of a specific receptor-dependent signaling pathway can be achieved and on how cross-talk between different second messenger cascades may contribute to fine-tune short- and long-term receptor activity.
The excitatory actions of glutamate in the central nervous system are mediated by two distinct classes of receptors: ionotropic and metabotropic. While ionotropic receptors drive fast neurotransmission, the stimulation of metabotropic glutamate receptors generates slower and longer lasting changes in the signaling cascades activated in neuronal and glial cells. Metabotropic glutamate receptors (mGluRs; mGluR1 through mGluR8) are classified into three distinct groups according to their sequence homology, pharmacological profile, and signaling properties; group I receptors are linked to phosphoinositide metabolism while groups II and III inhibit adenylyl cyclase (1, 2). Many functions have been ascribed to these receptors, such as regulation of neurotransmitter release, direct mediation of glutamatergic synaptic transmission, modulation of membrane excitability, and regulation of N-methyl-d-aspartate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and γ-aminobutyric acid type A receptor activity (1). This vast array of effects underscores the role of mGluRs in modulating synaptic plasticity and their neurotoxic as well as neuroprotective action (1, 3).
As for other G protein-coupled receptors (GPCRs), mGluRs induce diverse and long-lasting cellular responses; these actions depend on the effectors regulated by each second messenger molecule and on the cross-talk between signaling pathways. In the past several years, it has become increasingly clear that many GPCRs can initiate simultaneously multiple second messenger cascades because of their ability to couple to more than one G protein α subunit (4) and to the functional properties of the released βγ subunits (5). In addition, several new effector molecules interacting with GPCRs have been uncovered; often these functional associations appear to be direct and not secondary to G protein activation, thus disclosing unexpected physiological properties of the receptor superfamily (6). In the case of mGluR1α, the receptor has been shown to induce InsP3 accumulation and consequent release of Ca2+ from intracellular stores (7), accumulation of cAMP (7–9), and possibly activation of the mitogen-activated protein kinase pathway (10, 11). In addition, mGluR1α appears to regulate K+ (12) and Ca2+ (13, 14) channel opening and N-methyl-d-aspartate receptor activity (15), although the molecular mechanisms of these functional interactions are not clear at present.
Generally, GPCR responses desensitize rapidly, a process which is largely dependent on phosphorylation of either the receptor itself or other molecules engaged in the signaling cascade; in particular, for the β2-adrenergic receptor the molecular basis of homologous and heterologous desensitization have been partially deciphered (16–18). However, most of these studies have dealt with the analysis of linear signaling cascades, while fewer have addressed the question of how multiple receptor-dependent pathways are coordinately regulated (19–21).
Altogether, very little is known on how mGluR signaling is turned off, despite the fact that this is a critical step in vivo for receptor functions such as regulation of glutamate release (22). Very recently, it has been shown that overexpressed RGS proteins alter mGluR-mediated modulation of Ca2+ and K+ channels, a mechanism that may play a role in the regulation of receptor activity in pathological conditions (14, 23). On the other hand, in physiological conditions, mGluR-mediated stimulation of the InsP3 pathway desensitizes upon prolonged exposure to specific agonists (24–27) or following repeated stimulation (28–30). In these cases, protein kinase C (PKC) activation appears to mediate these effects.
In this study, we show that activation of PKC consequent to mGluR1α signaling via InsP3 induces a feedback inhibition of this pathway but does not affect receptor-mediated stimulation of cAMP. Conversely, activation of cAMP-dependent protein kinase (PKA) does not cause an attenuation of the receptor-mediated InsP3 response, but rather a potentiation. We provide evidence that this pathway-selective desensitization is achieved by the PKC-mediated phosphorylation of a threonine residue that is involved in receptor coupling to Gq/11 but not to Gs. Furthermore, we show that mutation of this residue, located in the second intracellular loop of mGluR1α, almost completely abolishes mGluR1α rapid desensitization induced by repeated exposure to l-glutamate (Glu) as well as receptor susceptibility to PKC activation.
Materials and Methods
Materials.
All of the reagent grade chemicals used in the described experiments were purchased from Sigma. T3 RNA polymerase and Expand High Fidelity Taq Polymerase were purchased from Roche Molecular Biochemicals; RNase inhibitor and Cap Analog from Ambion (Austin, TX); class I collagenase from Worthington; 4β-phorbol-12-myristate (PMA) and forskolin from Sigma; bisindolylmaleimide I from Alexis Biochemicals (San Diego, CA); and purified PKC from Calbiochem (La Jolla, CA). Tissue culture reagents were purchased from Life Technologies (Gaithersburg, MD). Human chorionic gonadotropin-tested Xenopus laevis females were obtained from Nasco (Fort Atkinson, WI).
Expression in HEK 293 Cells and Determination of Phosphoinositide Hydrolysis and cAMP Accumulation.
Cell culture, transfection experiments, and assays for the determination of total inositols and cAMP production were conducted as described previously (9).
Expression in Xenopus laevis Oocytes and Two-Electrode Voltage-Clamp Recordings.
Mutated variants of rat mGluR1α were generated as previously reported (9). Wild-type and mutant mGluR1α cloned into pBlueScript were linearized at the 3′ end with XhoI, digested with 2 mg ml−1 proteinase K, purified by phenol-chloroform extraction, and then used as template for in vitro RNA synthesis with T3 RNA polymerase, essentially as described (31). The RNA was phenol-chloroform extracted and its integrity was verified by fractionation on a 1% agarose-formaldehyde gel. To prepare oocytes for injection, ovary lobes were surgically removed from anesthetized Xenopus laevis mature females and defolliculated by digestion for 2 h at room temperature with 2 mg ml−1 collagenase. Stage VI oocytes were incubated for about 16 h at 18°C in Barth's saline (32) and microinjected with ≈5 ng of RNA dissolved in diethyl pyrocarbonate-treated water in a final volume of 50 nl. After a 3- to 4-day incubation at 18°C, the injected oocytes were voltage clamped at −60 mV using a Cornerstone TEV-200 amplifier (Dagan Instruments, Minneapolis). During the recording, oocytes were maintained at room temperature in Ringer's medium (109 mM NaCl, 1 mM KCl, 1 mM MgCl2, 2 mM CaCl2, and 5 mM Hepes, pH 7.2) in the perfusion chamber and challenged with 1 mM Glu in Ringer's medium delivered by gravity flow (≈1 ml min−1). Clamping electrodes were filled with 3 M KCl. The current tracings were collected on a chart recorder and digitized using a scanner for data presentation. Statistical analysis was performed with the program Instat 2.00 (GraphPad software, GraphPad, San Diego).
Bacterial Expression of Glutathione S-Transferase (GST)-mGluR1α Fusion Proteins.
Fusion proteins comprising GST at the amino terminus in-frame with the second intracellular loop (i2) of mGluR1α were generated by PCR and standard cloning techniques. The following oligonucleotides were used for amplification of the i2: 5′-CGTGGATCCCGTATTGCACGCATCCTGGCTGGCGCCAAGA-3′ (sense) and 5′-TTCCCGGGTCAGTGATGGTGATGGTGATGCACTTGGGAAAGC-3′ (antisense). Through this amplification, the i2 region was engineered to harbor an in-frame silent BamHI site at the amino end and a six-histidine tag at the carboxyl end. To prevent possible phosphorylation of Ser689 and Ser702, the S702R mutant (9) was used as template in the reaction and a point mutation was included in the sense primer to convert Ser689 to Ala. The PCR products were digested with BamHI and SmaI and subcloned into the pGEX-4T-2 vector (Amersham Pharmacia). The final construct was verified by sequencing. Following transformation into BL21(D3) Escherichia coli cells (Stratagene), clones expressing the fusion proteins were grown at 37°C in 2× YT broth and induced with 1 mM isopropyl-β-d-thiogalactopyranoside. Recombinant proteins were purified from the soluble fraction by binding onto glutathione-agarose columns and were eluted with 5 mM reduced glutathione. After extensive dialysis against 20 mM Tris-HCl (pH 7.4)/2 mM CaCl2, the protein content was determined by the Bio-Rad protein assay system (Bio-Rad).
In Vitro Phosphorylation Assay.
For the in vitro phosphorylation assays, 25 pmol of each purified fusion protein were incubated with the following reagents: 10 mM MgCl2, 0.5 mM CaCl2, 0.25% BSA, 0.5 mM dithioerythritol, 100 μg/ml phosphatidyl serine, 50 μM adenosine triphosphate, and 4.8 × 105 dpm [γ-32P]ATP (≈4 nmol per reaction; DuPont/NEN). The reaction, in a 25-μl final volume, was started by adding 0.01 U of PKC and incubated at 30°C for 20 min. Following the addition of SDS sample buffer, the phosphorylated proteins were separated on a 14% SDS-polyacrylamide gel; the gel was then fixed for 30 min with 10% acetic acid, dried, and exposed for autoradiography.
Results
Effect of PKC and PKA Activation on mGluR1α-Mediated Signaling.
In heterologous systems, mGluR1α can activate not only the InsP3/Ca2+ via Gq/11 but also the stimulatory cAMP pathway through interaction with Gs (7–9). Here, we used HEK 293 cells to express wild-type mGluR1α and to test the effect of PKC activation on both receptor-mediated pathways.
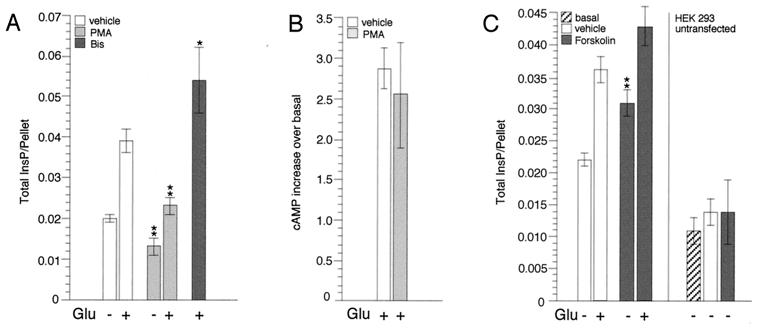
Activation of PKC was achieved by preincubating the transfected cells for 30 min with 1 μM PMA, a potent and specific PKC activator; following this treatment, cells were stimulated with Glu (1 mM) in the continued presence of PMA and total inositol phosphate (InsP) accumulation was determined. After incubation with PMA, both the constitutive (9, 33) and the Glu-stimulated activity of mGluR1α were decreased (Fig. 1A), indicating a role for PKC not only in agonist-dependent desensitization but also in the down-regulation of intrinsic receptor activity (9, 33). Consistently, inhibition of PKC with bisindolylmaleimide I (applied at 2 μM for 1 h and during stimulation with Glu) generated an increase in InsP accumulation (Fig. 1A). We then tested the effect of PKC activation on the other signaling pathway mediated by mGluR1α in this system, and measured the Glu-induced accumulation of cAMP following incubation with PMA. Results in Fig. 1B show that in this case PKC activation has no significant effect on receptor activity, thus indicating that the two signaling cascades are regulated independently by different molecular mechanisms.
Figure 1.
PKC and PKA regulate mGluR1α signaling. (A) Effect of PMA and bisindolylmaleimide I (Bis) on mGluR1α-dependent InsP accumulation. For each experiment, HEK 293 cells were transfected with 10 μg of mGluR1α and 10 μg of carrier DNA and seeded into six wells of a 24-well cluster. Stimulation was carried out in Hanks' saline solution (containing divalents and glucose) after clearing glutamate from the media with glutamic-pyruvic transaminase/pyruvate (9). The cells were treated with either Me2SO (vehicle) or test reagents and then stimulated with Glu. Results represent means ± SEM of duplicate or triplicate determinations obtained from at least two independent experiments (*, P ≤ 0.05; **, P ≤ 0.01; two-tailed t test). (B) Effect of PMA on mGluR1α-dependent cAMP accumulation. HEK 293 cells were transfected with 5 μg of mGluR1α along with 5 μg of Gs and 10 μg of carrier DNA and seeded into four wells of a 24-well cluster. The cells were preincubated with either Me2SO or PMA for 30 min and then stimulated with Glu for 20 min in the continued presence of the test reagents. Results represent means ± SEM of duplicate determinations obtained from three independent experiments. (C) Effect of forskolin on mGluR1α-dependent InsP accumulation. Cell transfection and treatment were conducted as described in A; in control experiments, 4 × 105 untransfected cells were plated for each well. Results represent means ± SEM of triplicate determinations obtained from three independent experiments; for untransfected cells, results shown are means ± SD of triplicate determinations, representative of two independent experiments.
Next, we assessed whether activation of PKA, the downstream effector of the cAMP pathway, had any effect on mGluR1α-mediated stimulation of the InsP3/Ca2+ pathway. PKA activation was achieved by incubating transfected and untransfected control cells for 30 min with 50 μM forskolin and then stimulating with Glu. Results shown in Fig. 1C indicated that PKA activation significantly potentiates mGluR1α action, in particular its intrinsic activity.
Selective Desensitization of the InsP3 Pathway Proceeds from PKC-Mediated Phosphorylation of Thr695 in the G Protein-Coupling Domain of mGluR1α.
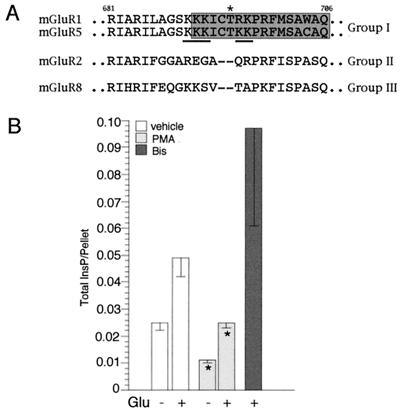
Prompted by the observation that PKC activation selectively desensitizes the InsP3 pathway following receptor stimulation, we sought to elucidate the molecular mechanisms of this effect. It has been shown that mGluR1α itself is substrate for PKC and that its level of phosphorylation increases upon stimulation with agonist (34). We reasoned that if PKC-mediated phosphorylation targeted residues within the receptor involved in the interaction with Gq/11 but not Gs, this could lead to the selective uncoupling from one signaling pathway. We have previously shown that Thr695 in the second intracellular loop of mGluR1α takes part in the interaction with Gq/11 but not with Gs (9). Thr695 is located within a hinge region that connects two putative α-helices and that is present only in group I mGluRs. This region is rich in basic amino acids and Thr695 falls within a consensus site for PKC-mediated phosphorylation (ref. 35; Fig. 2A). Together, these observations suggested that Thr695 could be a potential regulatory site of receptor activity, possibly involved in the selective desensitization of the InsP3/Ca2+ pathway.
Figure 2.
(A) Sequence alignment of the second intracellular loop of mGluRs; the region involved in selective coupling of mGluR1α with Gq/11 is boxed in gray. An asterisk highlights Thr695, which we propose to be substrate for PKC-mediated phosphorylation. Basic amino acid residues which can constitute the consensus for PKC are underlined. (B) Effect of PMA and bisindolylmaleimide I (Bis) on the Thr695Ala mutant receptor activity. Cell transfection and treatment were conducted as described for the wild-type receptor (Fig. 1A). Results represent means ± SEM of duplicate or triplicate determinations obtained from at least two independent experiments (*, P ≤ 0.05; two-tailed t test).
We first tested whether a mutant receptor in which Thr695 is substituted with an alanine (Thr695Ala), a residue that cannot be phosphorylated, differed from the wild-type receptor in its susceptibility to PKC in transfected HEK 293 cells. Following PMA treatment, both the constitutive and Glu-stimulated activity of the mutant receptor were decreased as for the wild-type (Fig. 2B). Consistently, incubation of the transfected cells with bisindolylmaleimide I caused an increase in total InsP accumulation (Fig. 2B). From this analysis, mutation of Thr695 did not appear to affect the receptor-mediated stimulation of the InsP3 pathway and the PKC-dependent desensitization. However, in this experimental paradigm, receptor activity is monitored over a long period and therefore rapid and transient changes in receptor responses that happen in neurons cannot be detected. In addition, constitutive activation along with the prolonged exposure to agonist are likely to induce a down-regulation of receptor activity that may mask transient alterations in the kinetic of the responses.
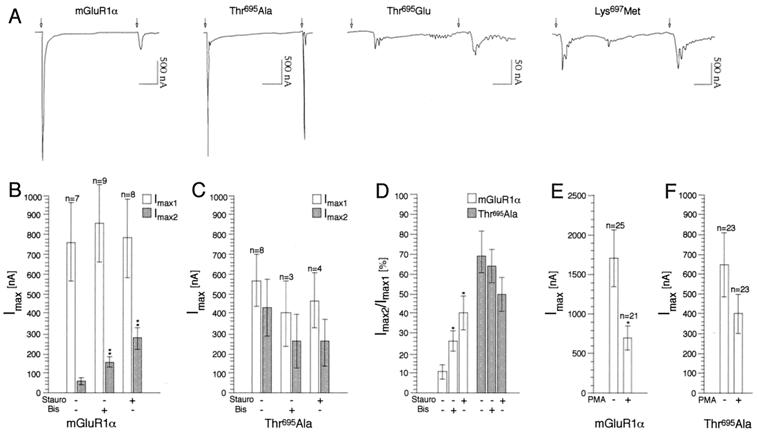
To examine whether phosphorylation at Thr695 could play a role in the rapid desensitization of receptor responses, we expressed wild-type and mutant receptors in Xenopus oocytes. In these cells, the release of intracellular Ca2+ induced by receptor stimulation causes the opening of Ca2+-activated Cl− channels, thus generating an inward current which is a measure of receptor activity. A 1-min pulse of Glu induced in mGluR1α-injected oocytes a large and transient current which rapidly goes back to baseline (Fig. 3A). Repeated applications of Glu after 1-, 2-, or 3-min intervals do not elicit responses (data not shown). After a 5-min washout with bath solution, a second application of Glu gave rise to a transient current of largely decreased amplitude compared with the first response (I2max/I1max = 0.17 ± 0.036, n = 23; Fig. 3 A, B, and D). To test whether desensitization of the receptor response was due to PKC activation, the injected oocytes were incubated with two different PKC inhibitors, staurosporin and bisindolylmaleimide I, for ≥20 min before stimulation with Glu. Both compounds caused a significant increase in the amplitude of the second response (Fig. 3 B and D), although they were not capable to fully prevent desensitization. Conversely, incubation with PMA at submaximal doses (100 nM for 10–20 min) before stimulation with Glu induced about 60% inhibition in current amplitude (Fig. 3E, control Imax = 1694 ± 360, n = 25; PMA-treated Imax = 686 ± 151, n = 21), confirming the role of PKC in regulating receptor desensitization.
Figure 3.
Rapid desensitization of mGluR1α depends on the phosphorylation state of Thr695. (A) Representative tracings of currents generated by wild-type and mutant receptors expressed in oocytes upon repeated application of Glu (1 mM); arrows indicate beginning of Glu application which lasted for 1 min. Time scale: 1 min. (B–F) PKC dependence of wild-type and mutant receptor responses. Injected oocytes were incubated with either staurosporin (100 nM) or bisindolylmaleimide I (2 μM) or PMA (100 nM) before recordings. Results are means ± SEM of values obtained from two independent experiments (*, P ≤ 0.05; **, P ≤ 0.01; two-tailed t test).
We then analyzed the responses evoked by two different mutant receptors in which Thr695 was replaced with either a neutral amino acid (Thr695Ala) or with a negatively charged residue (Thr695Glu), a mutation which mimics the effect produced by the addition of a phosphate group. A mutant was also tested in which the putative consensus for PKC-mediated phosphorylation is disrupted (Lys697Met; Fig. 2A). The Thr695Ala mutant evoked robust and transient currents comparable to the wild-type receptor (Fig. 3A). Interestingly, after a second challenge with agonist, the receptor showed only partial desensitization (I2max/I1max = 0.52 ± 0.08, n = 17; Fig. 3 A, C, and D). Neither staurosporin nor bisindolylmaleimide I have any significant effect on the amplitude of the second response (Fig. 3 C and D). In addition, incubation with PMA was much less effective (about 30%) in blocking receptor activity compared with wild type (Fig. 3F, control Imax = 646 ± 161, n = 23; PMA-treated Imax = 401 ± 95, n = 23). Together, these results demonstrate that the Thr695 residue is indeed critical for the Glu-induced receptor desensitization mediated by PKC. Mutant receptors harboring the Thr695Glu substitution gave rise to currents decreased in amplitude by an order of magnitude, slower in arising and returning to baseline. In addition, their waveform differed from wild type, being very often oscillatory: a representative current tracing obtained from this mutant is shown in Fig. 3A. These responses do not desensitize (I2max/I1max = 1.23 ± 0.28, n = 8). Finally, disruption of the PKC consensus sequence by the Lys697Met mutation generated currents slightly smaller in amplitude than those in wild type, partially oscillatory and with largely reduced desensitization (I2max/I1max = 0.65 ± 0.13, n = 15; Fig. 3A). For all receptors, a certain variability in the amplitude of the responses was observed when comparing experiments conducted with different batches of oocytes. However, the extent of desensitization was not correlated with the amplitude of the first response.
Thr695 Is a Substrate for PKC-Dependent Phosphorylation In Vitro.
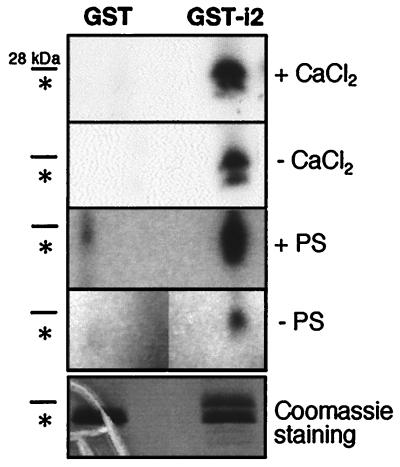
To test whether Thr695 can be a substrate for PKC-dependent phosphorylation, we generated a chimeric protein carrying GST fused to the second intracellular loop of mGluR1α (GST-i2). Point mutations were introduced at Ser689 and Ser702 of i2, so that the Thr695 residue was the only one available as substrate for PKC-mediated phosphorylation. The construct was expressed in bacteria and the corresponding recombinant protein was purified by affinity chromatography and incubated with PKC holoenzyme purified from brain. The results shown in Fig. 4 confirm that Thr695 is a substrate for PKC and the specificity of the reaction is further illustrated by its lipid and calcium dependence.
Figure 4.
Thr695 is phosphorylated in vitro. Recombinant fusion protein (GST-i2) and GST were used as substrate for phosphorylation by PKC purified from brain. Data shown are representative of three independent experiments. To verify for the specificity of the phosphorylation, the effects of lipids [phosphatidyl serine (PS)] and calcium on enzyme activity were tested. GST-i2 predicted molecular mass is 28 kDa; GST alone is 26 kDa and its position on the gel is indicated by an asterisk. The gels were exposed for autoradiography at −70°C for 12 h (with or without CaCl2) and 8 h (with or without PS), respectively.
Discussion
In recent years, our appreciation of the importance of intracellular signaling networks in shaping cellular responses has considerably increased (36). In analogy with other systems, we reasoned that the interplay between different signaling cascades is likely to have a critical role in determining the final output of mGluR activation. As an experimental paradigm to explore the possible cross-talk between different mGluR effectors, we selected two second messenger pathways activated in response to glutamate, InsP3/Ca2+ and cAMP, and examined their regulation by the respective downstream targets, PKC and PKA. Activation of the InsP3/Ca2+ pathway by group I mGluRs in neurons has been extensively characterized (1). In addition, stimulation of these receptors has been shown to increase cAMP levels during early developmental stages in the hippocampus (37), during the critical period in the visual cortex (38), and in neuronal cultures (39), although the molecular mechanisms underlying this effect are less well understood.
We found that PKC activation drastically curtails both the basal and Glu-stimulated activity of mGluR1α; however, under the same conditions, receptor-mediated stimulation of the cAMP pathway was not significantly altered. Feedback inhibition by PKC appears therefore to target selectively only mGluR1α signaling via InsP3. This opens the interesting possibility that receptor stimulation in neurons following neurotransmitter release could lead to the selective desensitization of this signaling pathway while leaving intact other receptor functions. Remarkably, such dual regulation of mGluR activity has been reported to take place both at presynaptic (30, 40) and postsynaptic sites (41). For example, activation of a phosphoinositide-linked mGluR at presynaptic terminals caused both facilitation and inhibition of glutamate release; of these two independent actions, only the signaling pathway that leads to facilitation desensitizes rapidly following PKC activation (40).
The effect of PKA on mGluR activity was more complex; treatment with forskolin potentiated rather than decreased receptor signaling via the InsP3/Ca2+ pathway, affecting in particular its basal activity. PKA appears therefore to have an opposing effect to PKC by inducing a sustained coupling of mGluR1α to the InsP3 pathway even in the absence of agonist. Several considerations arise from this observation: first, PKA action may target directly the receptor itself and/or indirectly other molecules involved in the InsP3 pathway. However, the fact that we did not observe a forskolin-induced increase in total inositols in control cells seems to point to a direct effect of the kinase on the receptor. Second, in neurons, simultaneous activation of other GPCRs linked to PKA activation may also lead to potentiation of mGluR1 responses by strengthening its signaling via InsP3.
To further understand the molecular mechanisms which govern mGluR function, we have attempted to clarify the nature of the structural determinants underlying the PKC-dependent pathway-selective desensitization. Wild-type mGluR1α was expressed in Xenopus oocytes and the kinetics of the receptor response following brief and repeated stimulations with agonist was examined. In this system, mGluR1α desensitized drastically and rapidly in response to glutamate. Desensitization ensued from PKC activation, since preincubation with specific PKC inhibitors partly rescued receptor responses. The fast and transient mGluR1α-induced currents recorded in oocytes correspond physiologically to the Ca2+ transients induced in other expression systems (42, 43) where PKC-dependent desensitization has also been reported (43). Thus, having available a system in which rapid and PKC-dependent changes in receptor activity can be analyzed, we set out to examine the effect of mutations of a putative PKC substrate, Thr695. Remarkably, substitution of the residue with Ala generated a mutant receptor that shows limited desensitization. Consistently, treatment with PKC inhibitors did not affect the mutant receptor responses. Together, these data indicate that Thr695 is an important target for PKC in the regulation of Glu-induced receptor activity. Thr695 was also replaced with Glu, a residue which by itself is negatively charged and hence mimics the modification which is introduced upon phosphorylation. Such strategy has proved useful in many cases to study the effects of phosphorylation with mutant molecules resembling constitutively phosphorylated substrates. In keeping with the prediction, the mutant Thr695Glu elicited responses greatly diminished in amplitude, as would be expected for a receptor “constitutively desensitized”; in addition, this mutant does not further desensitize following repeated stimulation with Glu. It is worth noting that all of the described mutations affect selectively InsP3/Ca2+ signaling, since the efficacy of the mutant receptors in stimulating the cAMP pathway is unaltered compared with wild type as previously shown (9).
On the basis of these observations, we propose a model for PKC action in which Thr695 is substrate for the kinase and acts as a molecular switch for mGluR1 activity. Upon ligand binding, a conformational change in the receptor is produced which would render the Thr695 residue accessible to phosphorylation. The added phosphate group would then prevent further coupling to Gq/11 but not to Gs. Interestingly, it has been recently reported that in cortical synaptosome preparations, PKC activation inhibits an mGluR-induced increase in GTPγS binding (44). This observation provides further evidence for a PKC-mediated uncoupling of mGluRs from their cognate G proteins. It has been shown that mGluR1α is phosphorylated in vivo both in the resting and agonist-bound conformation (34); however, the number and identity of the phosphorylated sites is still unknown. Our results suggest that different residues could be involved in controlling the basal or Glu-stimulated activity of the receptor, with Thr695 being a target for Glu-induced PKC-mediated phosphorylation.
Phosphoinositide-linked mGluRs, mGluR1 and 5, are generally regarded as functionally homologous because of the high similarity in their primary structure. However, recent reports have pointed out some interesting differences in the mode of intracellular Ca2 + release induced by these receptors. While mGluR1 induces single transient peaks of Ca2+, mGluR5 activation elicits oscillatory responses (42, 43). Such oscillations appear to be generated by PKC-dependent phosphorylation of a threonine residue which is present only in the carboxyl tail of mGluR5. Our work now reveals another difference in the regulation of the activity of these receptors. In fact, while in mGluR1 Thr695 plays a key role in regulating InsP3 signaling, mutation of the corresponding residue in mGluR5 generates inactive receptors (45). The different signaling properties and mechanisms of regulation of these receptors suggest that they subserve distinct functions in the neuronal environment.
Acknowledgments
We thank Drs. Lawrence G. Palmer and Han Choe (Weill Medical College) for help with the oocyte recordings. This work was supported by National Institutes of Health Grant EY09534 (to R.M.D.). A.F. was supported in part by the May and Samuel Rudin Family Foundation.
Abbreviations
- mGluR
metabotropic glutamate receptor
- GPCR
G protein-coupled receptor
- InsP3
inositol-1,4,5-trisphosphate
- PKC
protein kinase C
- PKA
protein kinase A
- GST
glutathione S-transferase
- i2
second intracellular loop
- InsP
inositol phosphate
References
- 1.Pin J-P, Duvoisin R M. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 2.Conn P J, Pin J-P. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 3.Nakanishi S. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 4.Gudermann, N. C., Gahwiler, B. H. & Schultz, G. (1996) Annu. Rev. Pharmacol. Toxicol.429–459. [DOI] [PubMed]
- 5.Clapham D E, Neer E J. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Hall R A, Premont R T, Lefkowitz R J. J Cell Biol. 1999;145:927–932. doi: 10.1083/jcb.145.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aramori I, Nakanishi S. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- 8.Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin J-P. J Neurosci. 1995;15:3970–3981. doi: 10.1523/JNEUROSCI.15-05-03970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francesconi A, Duvoisin R M. J Biol Chem. 1998;273:5615–5624. doi: 10.1074/jbc.273.10.5615. [DOI] [PubMed] [Google Scholar]
- 10.Roberson E D, English J D, Adams J P, Selcher J C, Kondratick C, Sweatt J D. J Neurosci. 1999;19:4337–4248. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraguti F, Baldani-Guerra B, Corsi M, Nakanishi S, Corti C. Eur J Neurosci. 1999;11:2073–2082. doi: 10.1046/j.1460-9568.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda S R, Lovinger D M, McCool B A, Lewis D L. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 13.McCool B A, Pin J-P, Harpold M M, Brust P F, Stauderman K A, Lovinger D M. J Neurophysiol. 1998;79:379–391. doi: 10.1152/jn.1998.79.1.379. [DOI] [PubMed] [Google Scholar]
- 14.Kammermeier P J, Ikeda S R. Neuron. 1999;22:819–829. doi: 10.1016/s0896-6273(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 15.Yu S P, Sensi S L, Canzoniero L M, Buisson A, Choi D W. J Physiol. 1997;499:721–732. doi: 10.1113/jphysiol.1997.sp021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hausdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 17.Pitcher J A, Freedman N J, Lefkowitz R J. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 18.Lefkowitz R J. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Yu L. J Biol Chem. 1994;269:7839–7842. [PubMed] [Google Scholar]
- 20.Daaka Y, Luttrell L M, Lefkowitz R J. Nature (London) 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Lee J W, Graves L M, Earp H S. EMBO J. 1998;17:2574–2583. doi: 10.1093/emboj/17.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrero I, Miras-Portugal M T, Sanchez-Prieto J. Nature (London) 1992;360:163–166. doi: 10.1038/360163a0. [DOI] [PubMed] [Google Scholar]
- 23.Saugstad J A, Marino M J, Folk J A, Helper J R, Conn P J. J Neurosci. 1998;18:905–913. doi: 10.1523/JNEUROSCI.18-03-00905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoepp D D, Johnson B G. Biochem Pharmacol. 1988;37:4299–4305. doi: 10.1016/0006-2952(88)90610-7. [DOI] [PubMed] [Google Scholar]
- 25.Manzoni O J, Finiels-Marlier F, Sassetti I, Bockaert J, LePeuch C, Sladeczek F. Neurosci Lett. 1990;109:146–151. doi: 10.1016/0304-3940(90)90553-l. [DOI] [PubMed] [Google Scholar]
- 26.Catania M V, Aronica E, Sortino M A, Canonico P L, Nicoletti F. J Neurochem. 1991;56:1329–1335. doi: 10.1111/j.1471-4159.1991.tb11429.x. [DOI] [PubMed] [Google Scholar]
- 27.Aronica E, Dell'Albani P, Condorelli D F, Nicoletti F, Hack N, Balazas R. Mol Pharmacol. 1994;44:981–989. [PubMed] [Google Scholar]
- 28.Herrero I, Miras-Portugal T, Sanchez-Prieto J. Eur J Neurosci. 1994;6:115–120. doi: 10.1111/j.1460-9568.1994.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 29.Guerineau N O, Bossu J-L, Gahwiler B H, Gerber U. J Physiol. 1997;500:487–496. doi: 10.1113/jphysiol.1997.sp022035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrero I, Miras-Portugal M T, Sanchez-Prieto J. J Biol Chem. 1998;273:1951–1958. doi: 10.1074/jbc.273.4.1951. [DOI] [PubMed] [Google Scholar]
- 31.Duvoisin R M, Deneris E S, Patrick J, Heinemann S F. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- 32.Coleman A. In: Translation of Eukaryotic Messenger RNA in Xenopus Oocytes. Hames B D, Higgins S J, editors. Oxford: IRL; 1984. pp. 271–302. [Google Scholar]
- 33.Prézeau L, Gomeza J, Ahern S, Mary S, Galvez T, Bockaert J, Pin J-P. Mol Pharmacol. 1996;49:422–429. [PubMed] [Google Scholar]
- 34.Alaluf S, Mulvihill E R, McIlhinney R A. FEBS Lett. 1995;367:301–305. doi: 10.1016/0014-5793(95)00575-t. [DOI] [PubMed] [Google Scholar]
- 35.Pearson R B, Kemp B E. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- 36.Bhalla U S, Iyengar R. Science. 1999;283:381. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- 37.Casabona G, Genazzani A A, DiStefano M, Sortino M A, Nicoletti F. J Neurochem. 1992;59:1161–1163. doi: 10.1111/j.1471-4159.1992.tb08360.x. [DOI] [PubMed] [Google Scholar]
- 38.Reid S N, Daw N W, Gregory D S, Flavin H. J Neurosci. 1996;16:7619–7626. doi: 10.1523/JNEUROSCI.16-23-07619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balazs R, Miller S, Chun Y, O'Toole J, Cotman C W. J Neurochem. 1998;70:2446–2458. doi: 10.1046/j.1471-4159.1998.70062446.x. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Moreno A, Sistiaga A, Lerma J, Sanchez-Prieto J. Neuron. 1998;21:1477–1486. doi: 10.1016/s0896-6273(00)80665-0. [DOI] [PubMed] [Google Scholar]
- 41.Fiorillo C D, Williams J T. Nature (London) 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- 42.Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. Nature (London) 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- 43.Kawabata S, Kohara A, Tsutsumi R, Itahana H, Hayashibe S, Yamaguchi T, Okada M. J Biol Chem. 1998;273:17381–17385. doi: 10.1074/jbc.273.28.17381. [DOI] [PubMed] [Google Scholar]
- 44.Macek T A, Schaffhauser H, Conn P J. J Neurosci. 1998;18:6138–6146. doi: 10.1523/JNEUROSCI.18-16-06138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gereau R W, Heinemann S F. Neuron. 1998;20:143–151. doi: 10.1016/s0896-6273(00)80442-0. [DOI] [PubMed] [Google Scholar]