Abstract
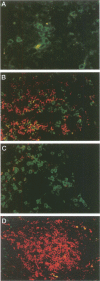
The pathogenesis of chronic hepatitis C and the mechanisms underlying progressive liver disease in patients with chronic hepatitis C infection are poorly understood. To demonstrate which inflammatory cells might be responsible for the necroinflammatory damage in chronic hepatitis C infection, we have correlated the phenotype of the intrahepatic lymphocytes and macrophages with histological activity in liver biopsy and explant specimens from 19 patients with chronic hepatitis C infection. In all stages of disease, more CD8+ than CD4+ lymphocytes were found. However, histologically active versus histologically mild hepatitis was associated with a trend toward greater parenchymal concentrations of CD4+ lymphocytes (0.71 +/- 0.27 per 10(4) microns 2 versus 0.35 +/- 0.15; not significant), significantly less parenchymal CD8+ lymphocytes (0.90 +/- 0.1 versus 1.70 +/- 0.3; t = 2.32, P = 0.03) and a greater parenchymal CD4/CD8 ratio (4.1 +/- 2.8 versus 0.91 +/- 0.3; t = 1.65, P = 0.07). No difference was found in the number of cells containing cytotoxic granules between the two groups. Greater numbers of CD4+ lymphocytes were found in liver biopsy specimens with little or no staining for hepatitis C virus antigen (1.47 +/- 0.88 versus 0.27 +/- 0.27; t = 2.28, P < 0.05). No significant differences were found in the macrophage subsets between the three stages of disease. Our data suggest that active histological disease in chronic hepatitis C infection may be associated with an increase in CD4+ lymphocytes and suggest that CD4+ T cells may play an important role in the hepatic injury in these patients.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter M. J., Margolis H. S., Krawczynski K., Judson F. N., Mares A., Alexander W. J., Hu P. Y., Miller J. K., Gerber M. A., Sampliner R. E. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992 Dec 31;327(27):1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- Ballardini G., Groff P., Pontisso P., Giostra F., Francesconi R., Lenzi M., Zauli D., Alberti A., Bianchi F. B. Hepatitis C virus (HCV) genotype, tissue HCV antigens, hepatocellular expression of HLA-A,B,C, and intercellular adhesion-1 molecules. Clues to pathogenesis of hepatocellular damage and response to interferon treatment in patients with chronic hepatitis C. J Clin Invest. 1995 May;95(5):2067–2075. doi: 10.1172/JCI117893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botarelli P., Brunetto M. R., Minutello M. A., Calvo P., Unutmaz D., Weiner A. J., Choo Q. L., Shuster J. R., Kuo G., Bonino F. T-lymphocyte response to hepatitis C virus in different clinical courses of infection. Gastroenterology. 1993 Feb;104(2):580–587. doi: 10.1016/0016-5085(93)90430-k. [DOI] [PubMed] [Google Scholar]
- Cerny A., McHutchison J. G., Pasquinelli C., Brown M. E., Brothers M. A., Grabscheid B., Fowler P., Houghton M., Chisari F. V. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J Clin Invest. 1995 Feb;95(2):521–530. doi: 10.1172/JCI117694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bisceglie A. M., Goodman Z. D., Ishak K. G., Hoofnagle J. H., Melpolder J. J., Alter H. J. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991 Dec;14(6):969–974. doi: 10.1016/0270-9139(91)90113-a. [DOI] [PubMed] [Google Scholar]
- Ferrari C., Valli A., Galati L., Penna A., Scaccaglia P., Giuberti T., Schianchi C., Missale G., Marin M. G., Fiaccadori F. T-cell response to structural and nonstructural hepatitis C virus antigens in persistent and self-limited hepatitis C virus infections. Hepatology. 1994 Feb;19(2):286–295. [PubMed] [Google Scholar]
- Freni M. A., Artuso D., Gerken G., Spanti C., Marafioti T., Alessi N., Spadaro A., Ajello A., Ferraù O. Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. Hepatology. 1995 Aug;22(2):389–394. [PubMed] [Google Scholar]
- Gonzalez-Peralta R. P., Davis G. L., Lau J. Y. Pathogenetic mechanisms of hepatocellular damage in chronic hepatitis C virus infection. J Hepatol. 1994 Aug;21(2):255–259. doi: 10.1016/s0168-8278(05)80405-2. [DOI] [PubMed] [Google Scholar]
- Hanabuchi S., Koyanagi M., Kawasaki A., Shinohara N., Matsuzawa A., Nishimura Y., Kobayashi Y., Yonehara S., Yagita H., Okumura K. Fas and its ligand in a general mechanism of T-cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4930–4934. doi: 10.1073/pnas.91.11.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K., Van Thiel D. H., Herberman R. B., Whiteside T. L. Natural killer activity of human liver-derived lymphocytes in various liver diseases. Hepatology. 1991 Sep;14(3):495–503. [PubMed] [Google Scholar]
- Hiramatsu N., Hayashi N., Katayama K., Mochizuki K., Kawanishi Y., Kasahara A., Fusamoto H., Kamada T. Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology. 1994 Jun;19(6):1354–1359. [PubMed] [Google Scholar]
- Hoffmann R. M., Diepolder H. M., Zachoval R., Zwiebel F. M., Jung M. C., Scholz S., Nitschko H., Riethmüller G., Pape G. R. Mapping of immunodominant CD4+ T lymphocyte epitopes of hepatitis C virus antigens and their relevance during the course of chronic infection. Hepatology. 1995 Mar;21(3):632–638. [PubMed] [Google Scholar]
- Hopf U., Möller B., Küther D., Stemerowicz R., Lobeck H., Lüdtke-Handjery A., Walter E., Blum H. E., Roggendorf M., Deinhardt F. Long-term follow-up of posttransfusion and sporadic chronic hepatitis non-A, non-B and frequency of circulating antibodies to hepatitis C virus (HCV). J Hepatol. 1990 Jan;10(1):69–76. doi: 10.1016/0168-8278(90)90075-3. [DOI] [PubMed] [Google Scholar]
- Ishak K., Baptista A., Bianchi L., Callea F., De Groote J., Gudat F., Denk H., Desmet V., Korb G., MacSween R. N. Histological grading and staging of chronic hepatitis. J Hepatol. 1995 Jun;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Koziel M. J., Dudley D., Afdhal N., Choo Q. L., Houghton M., Ralston R., Walker B. D. Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope proteins of HCV. J Virol. 1993 Dec;67(12):7522–7532. doi: 10.1128/jvi.67.12.7522-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczynski K., Beach M. J., Bradley D. W., Kuo G., di Bisceglie A. M., Houghton M., Reyes G. R., Kim J. P., Choo Q. L., Alter M. J. Hepatitis C virus antigen in hepatocytes: immunomorphologic detection and identification. Gastroenterology. 1992 Aug;103(2):622–629. doi: 10.1016/0016-5085(92)90856-t. [DOI] [PubMed] [Google Scholar]
- Lau J. Y., Davis G. L., Kniffen J., Qian K. P., Urdea M. S., Chan C. S., Mizokami M., Neuwald P. D., Wilber J. C. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet. 1993 Jun 12;341(8859):1501–1504. doi: 10.1016/0140-6736(93)90635-t. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minutello M. A., Pileri P., Unutmaz D., Censini S., Kuo G., Houghton M., Brunetto M. R., Bonino F., Abrignani S. Compartmentalization of T lymphocytes to the site of disease: intrahepatic CD4+ T cells specific for the protein NS4 of hepatitis C virus in patients with chronic hepatitis C. J Exp Med. 1993 Jul 1;178(1):17–25. doi: 10.1084/jem.178.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnier J. F., Degott C., Marcellin P., Hénin D., Erlinger S., Benhamou J. P. The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology. 1993 Mar;17(3):366–371. [PubMed] [Google Scholar]
- Mosnier J. F., Scoazec J. Y., Marcellin P., Degott C., Benhamou J. P., Feldmann G. Expression of cytokine-dependent immune adhesion molecules by hepatocytes and bile duct cells in chronic hepatitis C. Gastroenterology. 1994 Nov;107(5):1457–1468. doi: 10.1016/0016-5085(94)90550-9. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Norris A., Power C., Condez A., Burnes H., Schmekel B., Burke C. T cell dominated inflammatory reactions in the bronchioles of asymptomatic asthmatics are also present in the nasal mucosa. Postgrad Med J. 1991 Aug;67(790):747–753. doi: 10.1136/pgmj.67.790.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N., Enomoto N., Kurosaki M., Marumo F., Sato C. Sequential change of the hypervariable region of the hepatitis C virus genome in acute infection. J Med Virol. 1994 Jan;42(1):103–108. doi: 10.1002/jmv.1890420119. [DOI] [PubMed] [Google Scholar]
- Scheuer P. J., Ashrafzadeh P., Sherlock S., Brown D., Dusheiko G. M. The pathology of hepatitis C. Hepatology. 1992 Apr;15(4):567–571. doi: 10.1002/hep.1840150402. [DOI] [PubMed] [Google Scholar]
- Seeff L. B., Buskell-Bales Z., Wright E. C., Durako S. J., Alter H. J., Iber F. L., Hollinger F. B., Gitnick G., Knodell R. G., Perrillo R. P. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med. 1992 Dec 31;327(27):1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- Spiteri M. A., Poulter L. W. Characterization of immune inducer and suppressor macrophages from the normal human lung. Clin Exp Immunol. 1991 Jan;83(1):157–162. doi: 10.1111/j.1365-2249.1991.tb05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M., Urashima S., Takada A., Date T., Tanaka Y. Detection of antigens related to hepatitis C virus RNA encoding the NS5 region in the livers of patients with chronic type C hepatitis. Hepatology. 1994 Feb;19(2):265–272. [PubMed] [Google Scholar]
- Weiner A., Erickson A. L., Kansopon J., Crawford K., Muchmore E., Hughes A. L., Houghton M., Walker C. M. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2755–2759. doi: 10.1073/pnas.92.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]