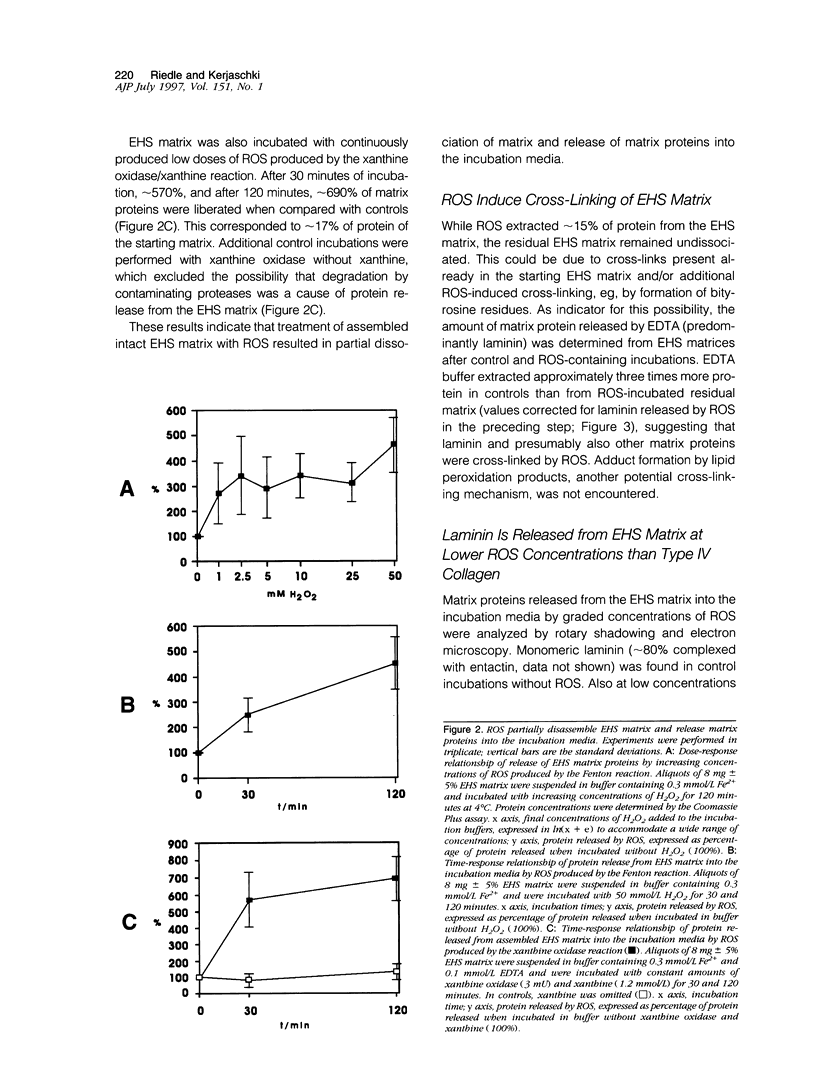
Abstract
Reactive oxygen species (ROS) are produced and released into the extracellular spaces in numerous diseases and contribute to development and progression, for example, of inflammatory diseases, proteinuria, and tumor invasion. However, little is known about ROS-induced chemical changes of interstitial matrix proteins and their consequences for the integrity of the matrix meshwork. As basement membranes and other matrices are highly cross-linked and complex, the relatively simple matrix produced by Engelbreth-Holm-Swarm (EHS) sarcoma, and proteins isolated therefrom, were incubated in vitro with defined concentrations of ROS that were generated by the Fenton or xanthine oxidase/xanthine reactions. This resulted in two counter-current effects. Although up to approximately 15% of the EHS matrix proteins were released into the supernatant in a ROS dose-response relationship, the residual insoluble matrix was partially cross-linked by ROS. Matrix proteins released into the supernatants were examined by rotary shadowing, quantitative sodium dodecyl sulfate polyacrylamide gel electrophoresis, immunoblotting, and fluorospectrometry for loss of tryptophans and formation of bityrosine residues. At relatively low ROS concentrations, selective liberation of morphologically intact laminin/entactin was found that, however, failed to reassociate and showed oxidative damage of its tryptophan residues. At higher ROS concentrations, laminin and entactin were progressively disintegrated, partially fragmented, and eventually completely degraded. At this point oligomers of type IV collagen predominated in the supernatant, and proteoglycans were not encountered at any concentration of ROS. Similar gradual molecular changes were also obtained when fractions of isolated soluble EHS matrix proteins were incubated with graded concentrations of ROS. In these experiments, the formation of covalently linked oligomers and aggregates paralleled the ROS-dependent formation of cross-linking bityrosine groups. ROS scavengers pinpointed to the hydroxyl radical as the most damaging radical species. Protease inhibitor experiments suggested that degradation of matrix proteins was caused primarily by the direct action of ROS and not by proteolysis by potentially contaminating proteases. Collectively, these results provide evidence that EHS matrix proteins show differential sensitivity to ROS-induced damage in a reproducible, sequential pattern, in the order entactin > laminin > type IV collagen, and that ROS cause partial dissociation and cross-linking of the EHS matrix.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aumailley M. Structure and supramolecular organization of basement membranes. Kidney Int Suppl. 1995 Jun;49:S4–S7. [PubMed] [Google Scholar]
- Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995 Oct;7(5):728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- Boyd-White J., Williams J. C., Jr Effect of cross-linking on matrix permeability. A model for AGE-modified basement membranes. Diabetes. 1996 Mar;45(3):348–353. doi: 10.2337/diab.45.3.348. [DOI] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton R. S. Metal-induced hepatotoxicity. Semin Liver Dis. 1996 Feb;16(1):3–12. doi: 10.1055/s-2007-1007214. [DOI] [PubMed] [Google Scholar]
- Bull C., Fee J. A., O'Neill P., Fielden E. M. Iron-ethylenediaminetetraacetic acid (EDTA)-catalyzed superoxide dismutation revisited: an explanation of why the dismutase activity of Fe-EDTA cannot be detected in the cytochrome c/Xanthine oxidase assay system. Arch Biochem Biophys. 1982 May;215(2):551–555. doi: 10.1016/0003-9861(82)90115-1. [DOI] [PubMed] [Google Scholar]
- Charonis A. S., Reger L. A., Dege J. E., Kouzi-Koliakos K., Furcht L. T., Wohlhueter R. M., Tsilibary E. C. Laminin alterations after in vitro nonenzymatic glycosylation. Diabetes. 1990 Jul;39(7):807–814. doi: 10.2337/diab.39.7.807. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Lin S. W., Pacifici R. E. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem. 1987 Jul 15;262(20):9914–9920. [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- Diamond J. R., Bonventre J. V., Karnovsky M. J. A role for oxygen free radicals in aminonucleoside nephrosis. Kidney Int. 1986 Feb;29(2):478–483. doi: 10.1038/ki.1986.24. [DOI] [PubMed] [Google Scholar]
- Engel J. Electron microscopy of extracellular matrix components. Methods Enzymol. 1994;245:469–488. doi: 10.1016/0076-6879(94)45024-2. [DOI] [PubMed] [Google Scholar]
- Girotti A. W., Thomas J. P., Jordan J. E. Xanthine oxidase-catalyzed crosslinking of cell membrane proteins. Arch Biochem Biophys. 1986 Dec;251(2):639–653. doi: 10.1016/0003-9861(86)90374-7. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem J. 1987 May 1;243(3):709–714. doi: 10.1042/bj2430709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Cross C. E. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994 Dec;102 (Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarasch E. D., Bruder G., Heid H. W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl. 1986;548:39–46. [PubMed] [Google Scholar]
- Kashihara N., Watanabe Y., Makino H., Wallner E. I., Kanwar Y. S. Selective decreased de novo synthesis of glomerular proteoglycans under the influence of reactive oxygen species. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6309–6313. doi: 10.1073/pnas.89.14.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Uchida K., Kawakishi S. Oxidative fragmentation of collagen and prolyl peptide by Cu(II)/H2O2. Conversion of proline residue to 2-pyrrolidone. J Biol Chem. 1992 Nov 25;267(33):23646–23651. [PubMed] [Google Scholar]
- Kerjaschki D., Neale T. J. Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis) J Am Soc Nephrol. 1996 Dec;7(12):2518–2526. doi: 10.1681/ASN.V7122518. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Partin J., Kaskel F. J. Changes in laminin during recovery from postischemic acute renal failure in rats. Exp Nephrol. 1996 Sep-Oct;4(5):279–285. [PubMed] [Google Scholar]
- Lonn E., Factor S. M., Van Hoeven K. H., Wen W. H., Zhao M., Dawood F., Liu P. Effects of oxygen free radicals and scavengers on the cardiac extracellular collagen matrix during ischemia-reperfusion. Can J Cardiol. 1994 Mar;10(2):203–213. [PubMed] [Google Scholar]
- Mackay A. R., Gomez D. E., Cottam D. W., Rees R. C., Nason A. M., Thorgeirsson U. P. Identification of the 72-kDa (MMP-2) and 92-kDa (MMP-9) gelatinase/type IV collagenase in preparations of laminin and Matrigel. Biotechniques. 1993 Dec;15(6):1048–1051. [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesel R., Kröger H., Kurpisz M., Weser U. Induction of arthritis in mice and rats by potassium peroxochromate and assessment of disease activity by whole blood chemiluminescence and 99mpertechnetate-imaging. Free Radic Res. 1995 Sep;23(3):213–227. doi: 10.3109/10715769509064035. [DOI] [PubMed] [Google Scholar]
- Mignatti P. Extracellular matrix remodeling by metalloproteinases and plasminogen activators. Kidney Int Suppl. 1995 Jun;49:S12–S14. [PubMed] [Google Scholar]
- Moseley R., Waddington R., Evans P., Halliwell B., Embery G. The chemical modification of glycosaminoglycan structure by oxygen-derived species in vitro. Biochim Biophys Acta. 1995 Jun 9;1244(2-3):245–252. doi: 10.1016/0304-4165(95)00010-9. [DOI] [PubMed] [Google Scholar]
- Noakes P. G., Gautam M., Mudd J., Sanes J. R., Merlie J. P. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995 Mar 16;374(6519):258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Nonaka Y., Iwagaki H., Kimura T., Fuchimoto S., Orita K. Effect of reactive oxygen intermediates on the in vitro invasive capacity of tumor cells and liver metastasis in mice. Int J Cancer. 1993 Jul 30;54(6):983–986. doi: 10.1002/ijc.2910540620. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Gehron P., McGoodwin E. B., Martin G. R., Valentine T., Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977 Jan 1;145(1):204–220. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasyuk A., Frati E., Ribault D., Mitrovic D. Effect of reactive oxygen species on the biosynthesis and structure of newly synthesized proteoglycans. Free Radic Biol Med. 1994 Feb;16(2):157–167. doi: 10.1016/0891-5849(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Price R. G., Taylor S. A., Crutcher E., Bergamaschi E., Franchini I., Mackie A. D. The assay of laminin fragments in serum and urine as an indicator of renal damage induced by toxins. Toxicol Lett. 1995 May;77(1-3):313–318. doi: 10.1016/0378-4274(95)03312-2. [DOI] [PubMed] [Google Scholar]
- Radeke H. H., Cross A. R., Hancock J. T., Jones O. T., Nakamura M., Kaever V., Resch K. Functional expression of NADPH oxidase components (alpha- and beta-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem. 1991 Nov 5;266(31):21025–21029. [PubMed] [Google Scholar]
- Richmond R., Halliwell B., Chauhan J., Darbre A. Superoxide-dependent formation of hydroxyl radicals: detection of hydroxyl radicals by the hydroxylation of aromatic compounds. Anal Biochem. 1981 Dec;118(2):328–335. doi: 10.1016/0003-2697(81)90590-x. [DOI] [PubMed] [Google Scholar]
- Riley D. P., Rivers W. J., Weiss R. H. Stopped-flow kinetic analysis for monitoring superoxide decay in aqueous systems. Anal Biochem. 1991 Aug 1;196(2):344–349. doi: 10.1016/0003-2697(91)90476-a. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Pou S., Ramos C. L., Cohen M. S., Britigan B. E. Free radicals and phagocytic cells. FASEB J. 1995 Feb;9(2):200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- Schnaper H. W., Kleinman H. K., Grant D. S. Role of laminin in endothelial cell recognition and differentiation. Kidney Int. 1993 Jan;43(1):20–25. doi: 10.1038/ki.1993.5. [DOI] [PubMed] [Google Scholar]
- Shah S. V. Evidence suggesting a role for hydroxyl radical in passive Heymann nephritis in rats. Am J Physiol. 1988 Mar;254(3 Pt 2):F337–F344. doi: 10.1152/ajprenal.1988.254.3.F337. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- Thakur V., Walker P. D., Shah S. V. Evidence suggesting a role for hydroxyl radical in puromycin aminonucleoside-induced proteinuria. Kidney Int. 1988 Oct;34(4):494–499. doi: 10.1038/ki.1988.208. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Jackson R. L. Lipid hydroperoxide involvement in copper-dependent and independent oxidation of low density lipoproteins. J Pharmacol Exp Ther. 1991 Mar;256(3):1182–1188. [PubMed] [Google Scholar]
- Tryggvason K. Mutations in type IV collagen genes and Alport phenotypes. Contrib Nephrol. 1996;117:154–171. doi: 10.1159/000424812. [DOI] [PubMed] [Google Scholar]
- Turner N., Mason P. J., Brown R., Fox M., Povey S., Rees A., Pusey C. D. Molecular cloning of the human Goodpasture antigen demonstrates it to be the alpha 3 chain of type IV collagen. J Clin Invest. 1992 Feb;89(2):592–601. doi: 10.1172/JCI115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton H. A., Byrne J., Robinson G. B. Studies of the permeation properties of glomerular basement membrane: cross-linking renders glomerular basement membrane permeable to protein. Biochim Biophys Acta. 1992 Mar 20;1138(3):173–183. doi: 10.1016/0925-4439(92)90035-l. [DOI] [PubMed] [Google Scholar]
- Weiner L., Kreimer D., Roth E., Silman I. Oxidative stress transforms acetylcholinesterase to a molten-globule-like state. Biochem Biophys Res Commun. 1994 Feb 15;198(3):915–922. doi: 10.1006/bbrc.1994.1130. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Winrow V. R., Winyard P. G., Morris C. J., Blake D. R. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993 Jul;49(3):506–522. doi: 10.1093/oxfordjournals.bmb.a072627. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Cheng Y. S., Colognato H. Laminin forms an independent network in basement membranes. J Cell Biol. 1992 Jun;117(5):1119–1133. doi: 10.1083/jcb.117.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Cheng Y. S. Laminin self-assembly: a three-arm interaction hypothesis for the formation of a network in basement membranes. Contrib Nephrol. 1994;107:47–56. doi: 10.1159/000422960. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C. Molecular architecture of basement membranes. FASEB J. 1990 Apr 1;4(6):1577–1590. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]