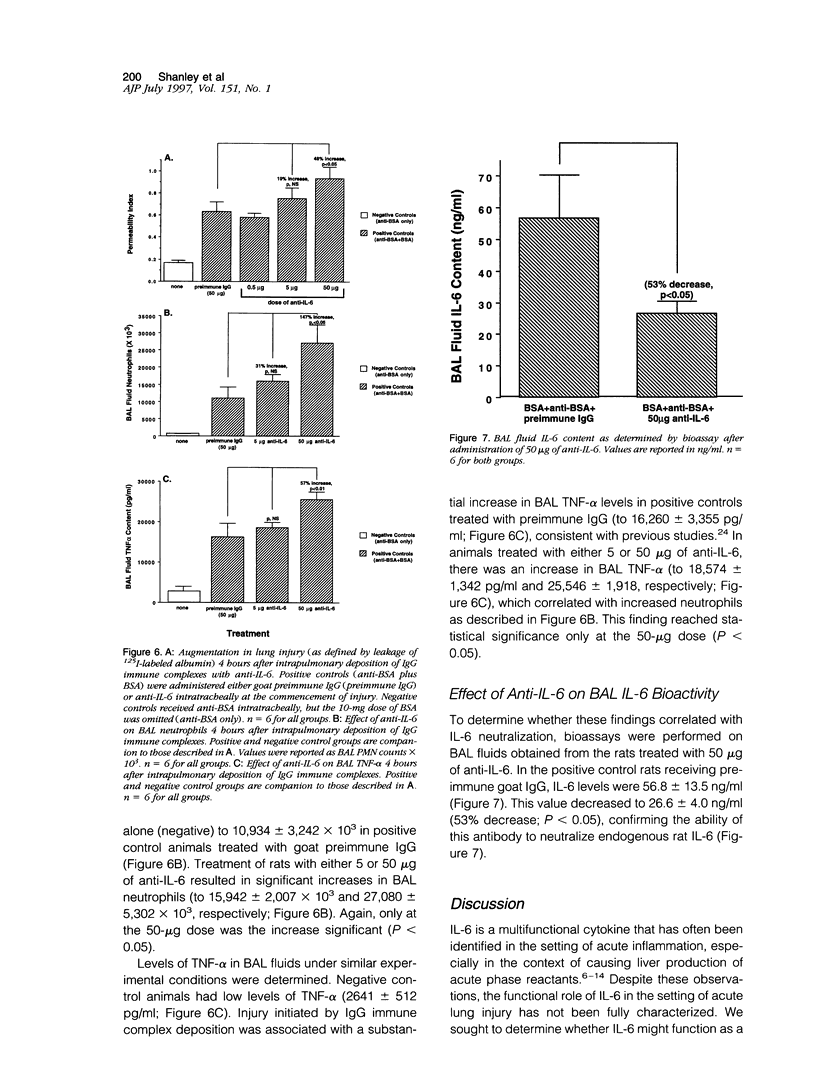
Abstract
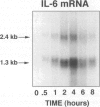
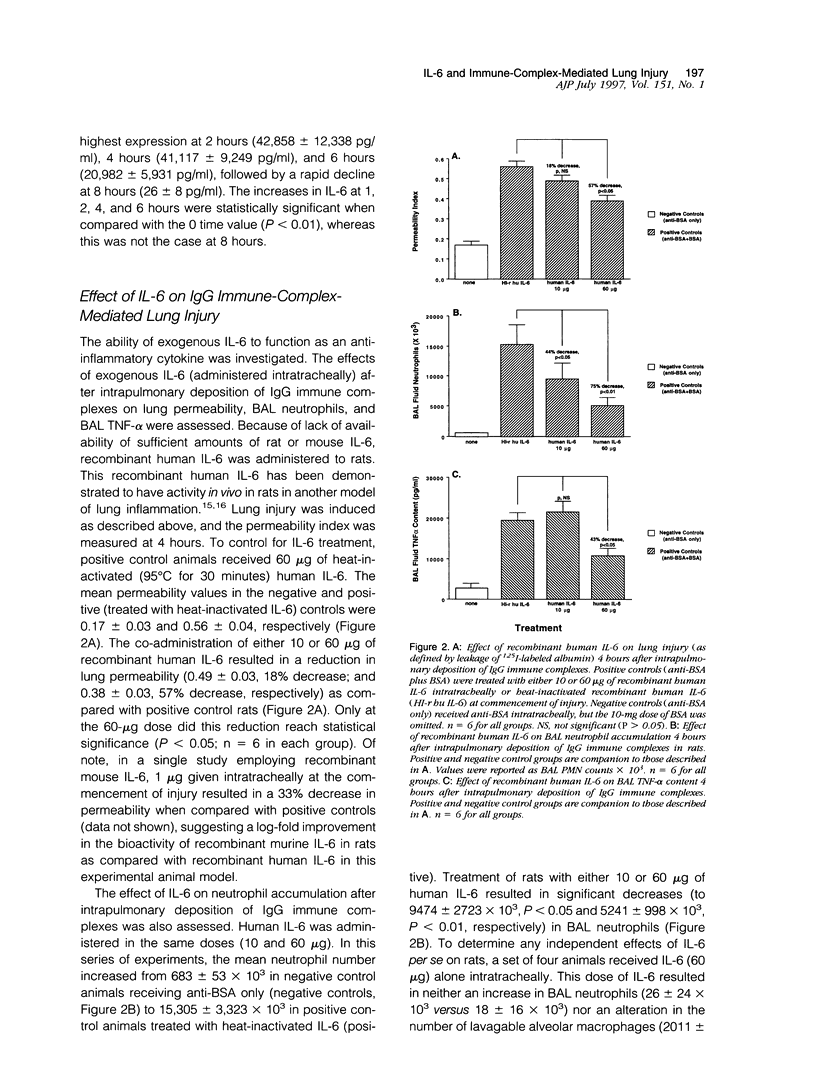
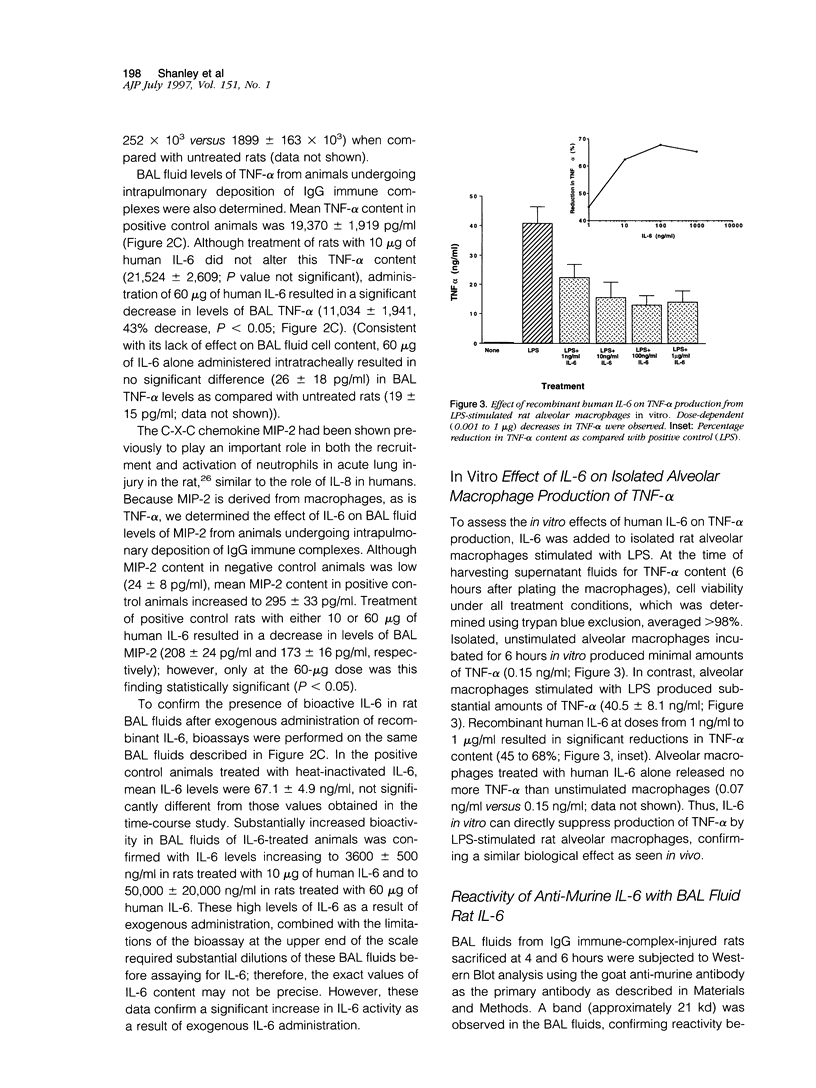
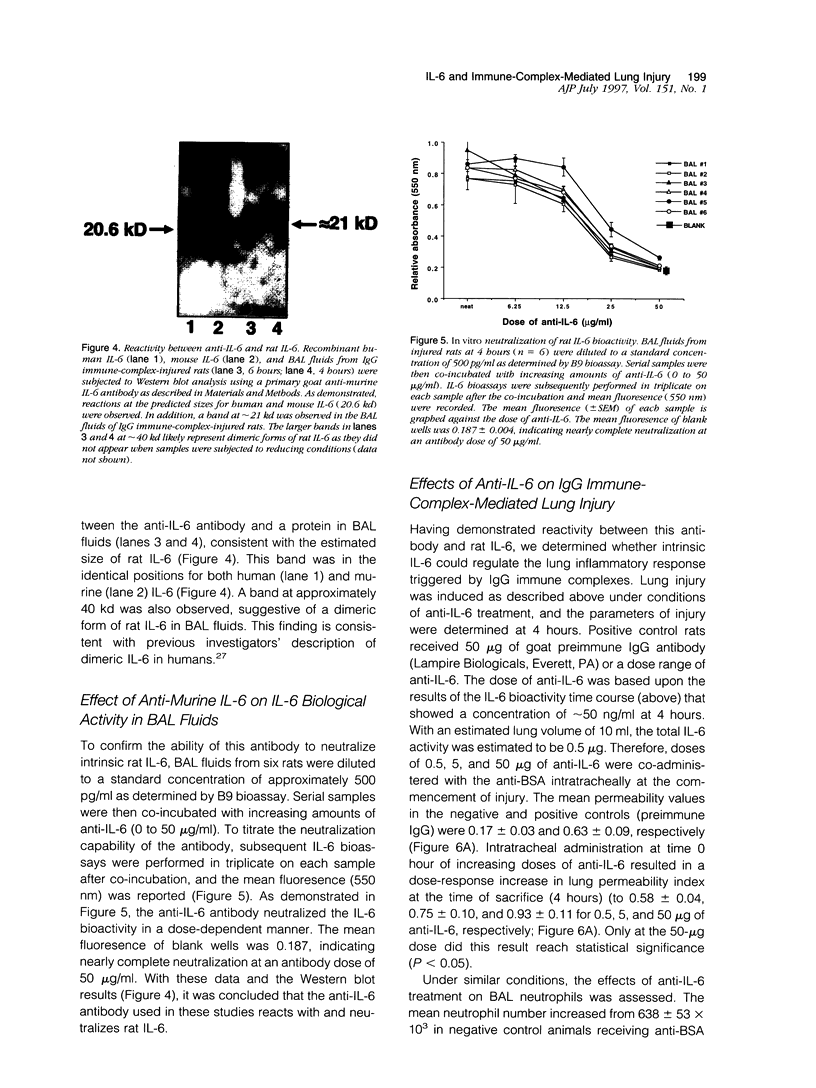
Interleukin-6 (IL-6) is a cytokine produced in response to a variety of inflammatory stimuli. Although IL-6 is often observed in increased amounts in acute respiratory distress syndrome, its role in the development of lung injury is unclear. The role of IL-6 was studied in the rat model of lung injury induced by the intra-alveolar deposition of IgG immune complexes. IL-6 induction, as determined by Northern blot analysis and bioactivity, was found as a function of time during the course of development of injury. Recombinant IL-6 instilled intratracheally at commencement of injury led to substantial reductions in lung vascular permeability, neutrophil accumulation, and levels of tumor necrosis factor (TNF)-alpha and macrophage inflammatory protein (MIP)-2 in bronchoalveolar lavage fluids. Conversely, blocking of intrinsic IL-6 by a neutralizing antibody resulted in increases in lung vascular permeability, neutrophil content, and TNF-alpha levels in bronchoalveolar lavage fluids. Rat alveolar macrophages stimulated in vitro with lipopolysaccharide in the presence of IL-6 showed a significant reduction in TNF-alpha expression. Together, these findings suggest that IL-6 acts as an intrinsic regulator of lung inflammatory injury after deposition of IgG immune complexes and that the protective effects of exogenously administered IL-6 may be in part linked to suppressed TNF-alpha production.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Alcorn J. M., Fierer J., Chojkier M. The acute-phase response protects mice from D-galactosamine sensitization to endotoxin and tumor necrosis factor-alpha. Hepatology. 1992 Jan;15(1):122–129. doi: 10.1002/hep.1840150121. [DOI] [PubMed] [Google Scholar]
- Ayala A., Wang P., Ba Z. F., Perrin M. M., Ertel W., Chaudry I. H. Differential alterations in plasma IL-6 and TNF levels after trauma and hemorrhage. Am J Physiol. 1991 Jan;260(1 Pt 2):R167–R171. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- Barton B. E., Jackson J. V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993 Apr;61(4):1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton B. E., Shortall J., Jackson J. V. Interleukins 6 and 11 protect mice from mortality in a staphylococcal enterotoxin-induced toxic shock model. Infect Immun. 1996 Mar;64(3):714–718. doi: 10.1128/iai.64.3.714-718.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell J. V., Andus T., Kunz D., Heinrich P. C. Interleukin-6. The major regulator of acute-phase protein synthesis in man and rat. Ann N Y Acad Sci. 1989;557:87–101. [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Andus T., Geiger T., Trullenque R., Fabra R., Heinrich P. C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989 Jan 2;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Damas P., Ledoux D., Nys M., Vrindts Y., De Groote D., Franchimont P., Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992 Apr;215(4):356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Interleukin-6 in mouse hypersensitivity pneumonitis: changes in lung free cells following depletion of endogenous IL-6 or direct administration of IL-6. J Leukoc Biol. 1992 Aug;52(2):197–201. doi: 10.1002/jlb.52.2.197. [DOI] [PubMed] [Google Scholar]
- Donnelly T. J., Meade P., Jagels M., Cryer H. G., Law M. M., Hugli T. E., Shoemaker W. C., Abraham E. Cytokine, complement, and endotoxin profiles associated with the development of the adult respiratory distress syndrome after severe injury. Crit Care Med. 1994 May;22(5):768–776. doi: 10.1097/00003246-199405000-00010. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Gennari R., Alexander J. W. Anti-interleukin-6 antibody treatment improves survival during gut-derived sepsis in a time-dependent manner by enhancing host defense. Crit Care Med. 1995 Dec;23(12):1945–1953. doi: 10.1097/00003246-199512000-00002. [DOI] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Taga T., Nakano N., Yasukawa K., Kashiwamura S., Shimizu K., Nakajima K., Pyun K. H., Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci U S A. 1985 Aug;82(16):5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch R. C., Rodriguez R., Manning T., Bishop M., Mead P., Shoemaker W. C., Abraham E. Effects of accidental trauma on cytokine and endotoxin production. Crit Care Med. 1993 Jun;21(6):839–845. doi: 10.1097/00003246-199306000-00010. [DOI] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablons D. M., Mulé J. J., McIntosh J. K., Sehgal P. B., May L. T., Huang C. M., Rosenberg S. A., Lotze M. T. IL-6/IFN-beta-2 as a circulating hormone. Induction by cytokine administration in humans. J Immunol. 1989 Mar 1;142(5):1542–1547. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meduri G. U., Headley S., Kohler G., Stentz F., Tolley E., Umberger R., Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995 Apr;107(4):1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Desrochers P. E., Chinnaiyan A. M., Gibbs D. F., Varani J., Johnson K. J., Weiss S. J. In vivo suppression of immune complex-induced alveolitis by secretory leukoproteinase inhibitor and tissue inhibitor of metalloproteinases 2. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11523–11527. doi: 10.1073/pnas.90.24.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Vaporciyan A. A., Miyasaka M., Tamatani T., Ward P. A. Tumor necrosis factor alpha regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol. 1993 Jun;142(6):1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Varani J., Dame M. K., Lane C. L., Smith C. W., Anderson D. C., Ward P. A. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991 Oct;88(4):1396–1406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Ward P. A. Immune complex-induced lung and dermal vascular injury. Differing requirements for tumor necrosis factor-alpha and IL-1. J Immunol. 1992 Jul 1;149(1):331–339. [PubMed] [Google Scholar]
- Mulligan M. S., Wilson G. P., Todd R. F., Smith C. W., Anderson D. C., Varani J., Issekutz T. B., Miyasaka M., Tamatani T., Myasaka M. Role of beta 1, beta 2 integrins and ICAM-1 in lung injury after deposition of IgG and IgA immune complexes. J Immunol. 1993 Mar 15;150(6):2407–2417. [PubMed] [Google Scholar]
- Northemann W., Braciak T. A., Hattori M., Lee F., Fey G. H. Structure of the rat interleukin 6 gene and its expression in macrophage-derived cells. J Biol Chem. 1989 Sep 25;264(27):16072–16082. [PubMed] [Google Scholar]
- Roumen R. M., Hendriks T., van der Ven-Jongekrijg J., Nieuwenhuijzen G. A., Sauerwein R. W., van der Meer J. W., Goris R. J. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993 Dec;218(6):769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter B., König B., Bergmann U., Müller F. E., König W. Interleukin 6--a potential mediator of lethal sepsis after major thermal trauma: evidence for increased IL-6 production by peripheral blood mononuclear cells. J Trauma. 1991 Dec;31(12):1663–1670. doi: 10.1097/00005373-199112000-00017. [DOI] [PubMed] [Google Scholar]
- Schmal H., Shanley T. P., Jones M. L., Friedl H. P., Ward P. A. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol. 1996 Mar 1;156(5):1963–1972. [PubMed] [Google Scholar]
- Shanley T. P., Schmal H., Friedl H. P., Jones M. L., Ward P. A. Regulatory effects of intrinsic IL-10 in IgG immune complex-induced lung injury. J Immunol. 1995 Apr 1;154(7):3454–3460. [PubMed] [Google Scholar]
- Svoboda P., Kantorová I., Ochmann J. Dynamics of interleukin 1, 2, and 6 and tumor necrosis factor alpha in multiple trauma patients. J Trauma. 1994 Mar;36(3):336–340. doi: 10.1097/00005373-199403000-00009. [DOI] [PubMed] [Google Scholar]
- Szalai A. J., Briles D. E., Volanakis J. E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J Immunol. 1995 Sep 1;155(5):2557–2563. [PubMed] [Google Scholar]
- Takai Y., Wong G. G., Clark S. C., Burakoff S. J., Herrmann S. H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):508–512. [PubMed] [Google Scholar]
- Ulich T. R., Guo K. Z., Remick D., del Castillo J., Yin S. M. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991 Apr 1;146(7):2316–2323. [PubMed] [Google Scholar]
- Ulich T. R., Yin S., Guo K., Yi E. S., Remick D., del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am J Pathol. 1991 May;138(5):1097–1101. [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Remick D. G., Kunkel S. L., Chensue S. W., Kunkel R. G., Johnson K. J., Ward P. A. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest. 1989 Dec;84(6):1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]