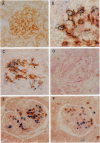
Abstract
We examined the functional role of interleukin (IL)-1 in mesangial cell proliferation during rat anti-Thy-1 nephritis by blocking its action with IL-1 receptor antagonist (IL-1ra). Anti-Thy-1 nephritis was induced by intravenous injection of 5 mg/kg OX-7 IgG (day 0) into inbred Wistar rats. Groups of animals (n = 9) were implanted with a micro-osmotic pump on day -1, which delivered 25 micrograms/hour human recombinant IL-1ra or saline continuously until the rats were killed at day 6, the peak of mesangial cell proliferation. Immunostaining showed that IL-1 was expressed by mesangial cells during disease. IL-1ra treatment did not affect the mild, but significant, proteinuria seen after OX-7 injection. Compared with saline treatment, IL-1ra treatment reduced mesangial cell proliferation (decreases 24% P < 0.05), glomerular hypercellularity (decreases 29%; P < 0.05), and glomerular macrophage accumulation (decreases 20%; P < 0.05). However, IL-1ra treatment had no effect on glomerular IL-1 beta mRNA expression and caused only a small reduction in the high levels of glomerular expression of platelet-derived growth factor-beta protein (decreases 6%; P < 0.05). IL-1ra caused a modest reduction in the marked up-regulation of glomerular transforming growth factor-beta 1 mRNA expression on day 6 (decreases 26%; P < 0.05), although urinary excretion of this factor was unaffected. Interestingly, IL-1ra treatment had relatively little effect upon glomerular deposition of laminin, fibronectin, and collagen type IV seen in this acute disease. In conclusion, this study has 1) demonstrated that IL-1 is expressed by mesangial cells in vivo, 2) demonstrated that IL-1 is a mesangial cell growth factor in experimental mesangioproliferative nephritis, and 3) suggests that IL-1 has little or no fibrogenic activity in mesangial matrix deposition.
Full text
PDF


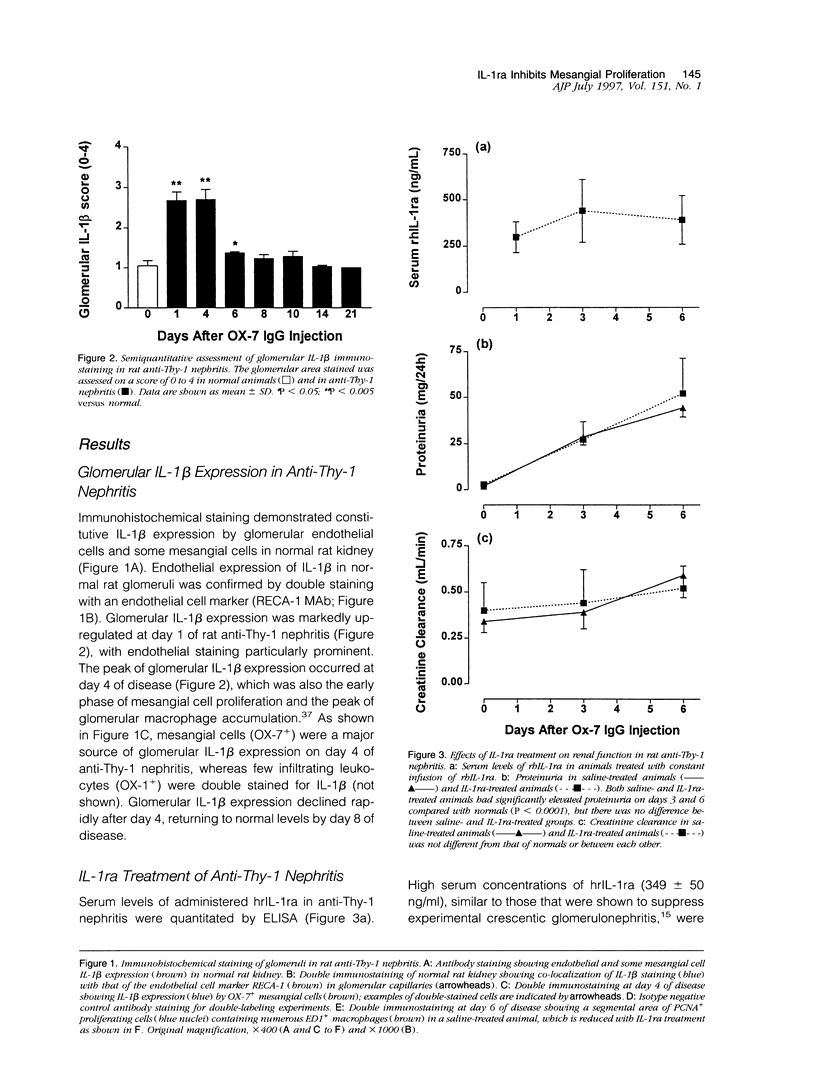
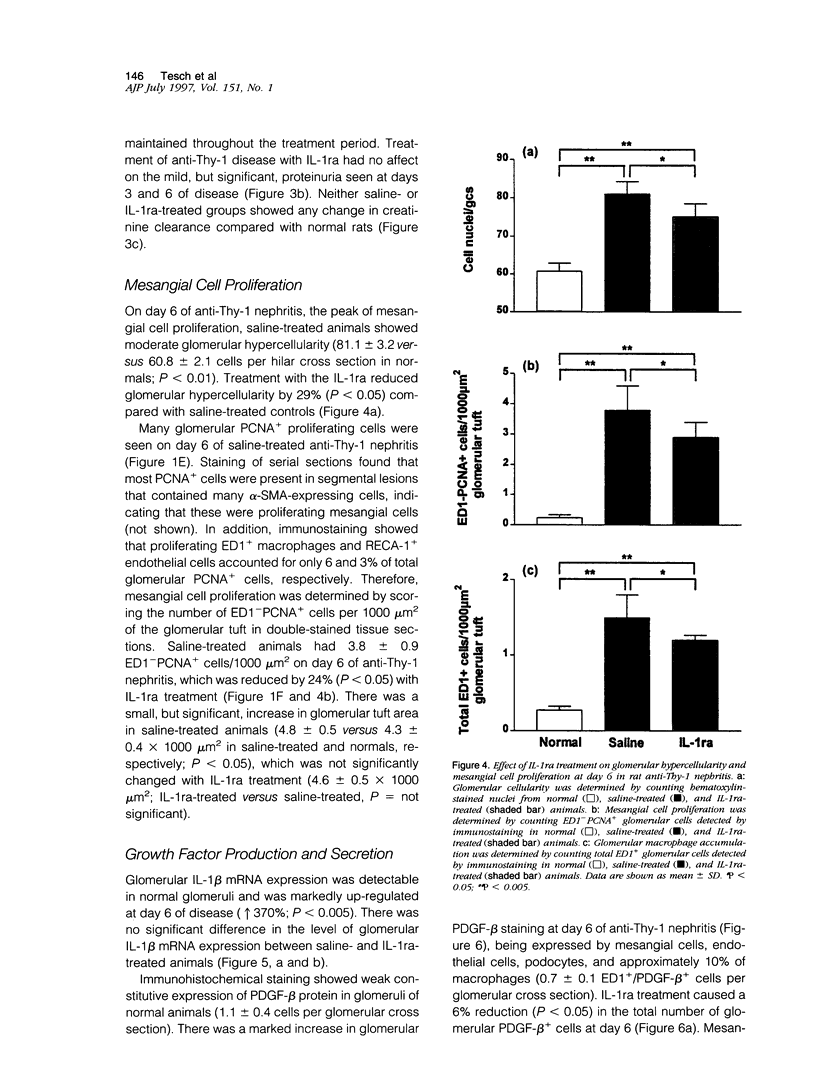
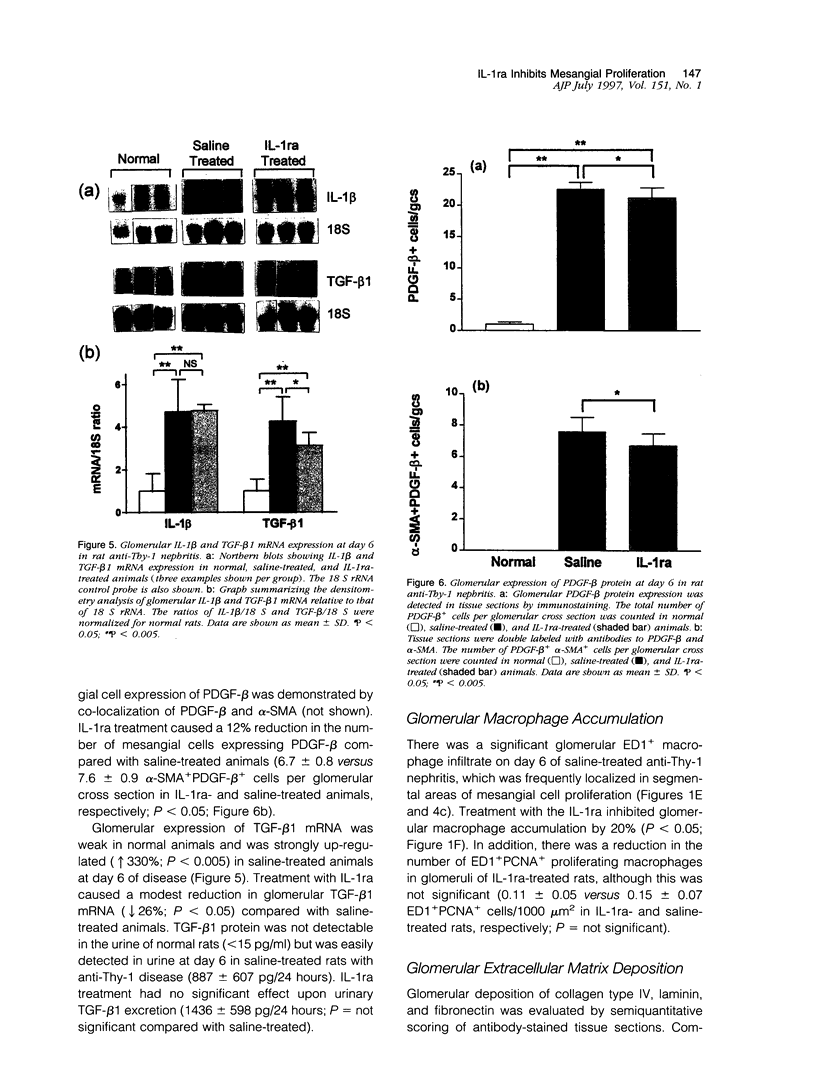
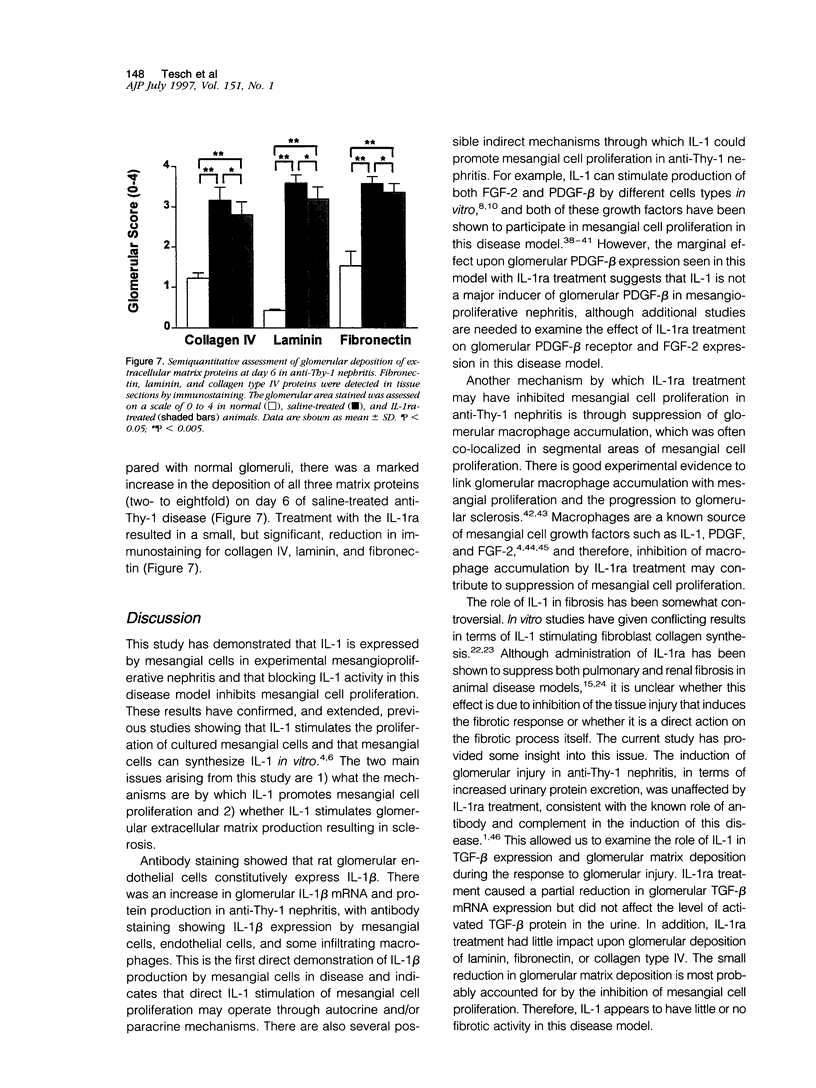


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott F., Ryan J. J., Ceska M., Matsushima K., Sarraf C. E., Rees A. J. Interleukin-1 beta stimulates human mesangial cells to synthesize and release interleukins-6 and -8. Kidney Int. 1991 Oct;40(4):597–605. doi: 10.1038/ki.1991.250. [DOI] [PubMed] [Google Scholar]
- Bathon J. M., Hwang J. J., Shin L. H., Precht P. A., Towns M. C., Horton W. E., Jr Type VI collagen-specific messenger RNA is expressed constitutively by cultured human synovial fibroblasts and is suppressed by interleukin-1. Arthritis Rheum. 1994 Sep;37(9):1350–1356. doi: 10.1002/art.1780370913. [DOI] [PubMed] [Google Scholar]
- Boswell J. M., Yui M. A., Burt D. W., Kelley V. E. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988 Nov 1;141(9):3050–3054. [PubMed] [Google Scholar]
- Diamond J. R., Pesek-Diamond I. Sublethal X-irradiation during acute puromycin nephrosis prevents late renal injury: role of macrophages. Am J Physiol. 1991 Jun;260(6 Pt 2):F779–F786. doi: 10.1152/ajprenal.1991.260.6.F779. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn A. M., van Goor H., Klatter F., Majoor G. D., van Bussel E., van Breda Vriesman P. J. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992 Apr;66(4):459–466. [PubMed] [Google Scholar]
- Floege J., Burns M. W., Alpers C. E., Yoshimura A., Pritzl P., Gordon K., Seifert R. A., Bowen-Pope D. F., Couser W. G., Johnson R. J. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992 Feb;41(2):297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- Floege J., Eng E., Lindner V., Alpers C. E., Young B. A., Reidy M. A., Johnson R. J. Rat glomerular mesangial cells synthesize basic fibroblast growth factor. Release, upregulated synthesis, and mitogenicity in mesangial proliferative glomerulonephritis. J Clin Invest. 1992 Dec;90(6):2362–2369. doi: 10.1172/JCI116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Alpers C. E., Barrett T. B., Bowen-Pope D. F., Johnson R. J. Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest. 1993 Dec;92(6):2952–2962. doi: 10.1172/JCI116918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Gordon K., Iida H., Pritzl P., Yoshimura A., Campbell C., Alpers C. E., Couser W. G. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int. 1991 Sep;40(3):477–488. doi: 10.1038/ki.1991.235. [DOI] [PubMed] [Google Scholar]
- Francki A., Uciechowski P., Floege J., von der Ohe J., Resch K., Radeke H. H. Autocrine growth regulation of human glomerular mesangial cells is primarily mediated by basic fibroblast growth factor. Am J Pathol. 1995 Nov;147(5):1372–1382. [PMC free article] [PubMed] [Google Scholar]
- Henke C., Marineili W., Jessurun J., Fox J., Harms D., Peterson M., Chiang L., Doran P. Macrophage production of basic fibroblast growth factor in the fibroproliferative disorder of alveolar fibrosis after lung injury. Am J Pathol. 1993 Oct;143(4):1189–1199. [PMC free article] [PubMed] [Google Scholar]
- Iida H., Seifert R., Alpers C. E., Gronwald R. G., Phillips P. E., Pritzl P., Gordon K., Gown A. M., Ross R., Bowen-Pope D. F. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6560–6564. doi: 10.1073/pnas.88.15.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Raines E. W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992 May 1;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991 Jan;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- Lan H. Y., Mu W., Nikolic-Paterson D. J., Atkins R. C. A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem. 1995 Jan;43(1):97–102. doi: 10.1177/43.1.7822770. [DOI] [PubMed] [Google Scholar]
- Lan H. Y., Nikolic-Paterson D. J., Zarama M., Vannice J. L., Atkins R. C. Suppression of experimental crescentic glomerulonephritis by the interleukin-1 receptor antagonist. Kidney Int. 1993 Feb;43(2):479–485. doi: 10.1038/ki.1993.70. [DOI] [PubMed] [Google Scholar]
- Larsen K. Creatinine assay by a reaction-kinetic principle. Clin Chim Acta. 1972 Oct;41:209–217. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- Lonnemann G., Shapiro L., Engler-Blum G., Müller G. A., Koch K. M., Dinarello C. A. Cytokines in human renal interstitial fibrosis. I. Interleukin-1 is a paracrine growth factor for cultured fibrosis-derived kidney fibroblasts. Kidney Int. 1995 Mar;47(3):837–844. doi: 10.1038/ki.1995.126. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Larsen A. Cell cycle-dependent interleukin 1 gene expression by cultured glomerular mesangial cells. J Clin Invest. 1988 Jul;82(1):115–122. doi: 10.1172/JCI113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. Stimulation of rat mesangial cell proliferation by macrophage interleukin 1. J Immunol. 1983 Dec;131(6):2830–2836. [PubMed] [Google Scholar]
- Lovett D. H., Szamel M., Ryan J. L., Sterzel R. B., Gemsa D., Resch K. Interleukin 1 and the glomerular mesangium. I. Purification and characterization of a mesangial cell-derived autogrowth factor. J Immunol. 1986 May 15;136(10):3700–3705. [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Atkins R. C. Glomerular cells and macrophages in the progression of experimental focal and segmental glomerulosclerosis. Am J Pathol. 1989 Apr;134(4):933–945. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K. Production of interleukin-1 by glomerular macrophages in nephrotoxic serum nephritis. Am J Nephrol. 1990;10(6):502–506. doi: 10.1159/000168176. [DOI] [PubMed] [Google Scholar]
- Nikolic-Paterson D. J., Jun Z., Tesch G. H., Lan H. Y., Foti R., Atkins R. C. De novo CD44 expression by proliferating mesangial cells in rat anti-Thy-1 nephritis. J Am Soc Nephrol. 1996 Jul;7(7):1006–1014. doi: 10.1681/ASN.V771006. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Vesin C., Grau G. E., Thompson R. C. Interleukin 1 receptor antagonist (IL-1ra) prevents or cures pulmonary fibrosis elicited in mice by bleomycin or silica. Cytokine. 1993 Jan;5(1):57–61. doi: 10.1016/1043-4666(93)90024-y. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Raghow R., Stricklin G. P., Poppleton H., Seyer J. M., Kang A. H. Modulation of fibroblast functions by interleukin 1: increased steady-state accumulation of type I procollagen messenger RNAs and stimulation of other functions but not chemotaxis by human recombinant interleukin 1 alpha and beta. J Cell Biol. 1988 Feb;106(2):311–318. doi: 10.1083/jcb.106.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S. W., Kondaiah P., Roberts A. B., Sporn M. B. cDNA cloning by PCR of rat transforming growth factor beta-1. Nucleic Acids Res. 1990 May 25;18(10):3059–3059. doi: 10.1093/nar/18.10.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Richardson C. A., Gordon K. L., Couser W. G., Bomsztyk K. IL-1 beta increases laminin B2 chain mRNA levels and activates NF-kappa B in rat glomerular epithelial cells. Am J Physiol. 1995 Feb;268(2 Pt 2):F273–F278. doi: 10.1152/ajprenal.1995.268.2.F273. [DOI] [PubMed] [Google Scholar]
- Schotanus K., Holtkamp G. M., Meloen R. H., Puijk W. C., Berkenbosch F., Tilders F. J. Domains of rat interleukin 1 beta involved in type I receptor binding. Endocrinology. 1995 Jan;136(1):332–339. doi: 10.1210/endo.136.1.7530194. [DOI] [PubMed] [Google Scholar]
- Sterzel R. B., Schulze-Lohoff E., Marx M. Cytokines and mesangial cells. Kidney Int Suppl. 1993 Jan;39:S26–S31. [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Takemura T., Yoshioka K., Murakami K., Akano N., Okada M., Aya N., Maki S. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994;424(5):459–464. doi: 10.1007/BF00191429. [DOI] [PubMed] [Google Scholar]
- Torbohm I., Berger B., Schönermark M., von Kempis J., Rother K., Hänsch G. M. Modulation of collagen synthesis in human glomerular epithelial cells by interleukin 1. Clin Exp Immunol. 1989 Mar;75(3):427–431. [PMC free article] [PubMed] [Google Scholar]
- Wangoo A., Taylor I. K., Haynes A. R., Shaw R. J. Up-regulation of alveolar macrophage platelet-derived growth factor-B (PDGF-B) mRNA by interferon-gamma from Mycobacterium tuberculosis antigen (PPD)-stimulated lymphocytes. Clin Exp Immunol. 1993 Oct;94(1):43–50. doi: 10.1111/j.1365-2249.1993.tb05975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem N. H., Lane D. P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990 May;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Werber H. I., Emancipator S. N., Tykocinski M. L., Sedor J. R. The interleukin 1 gene is expressed by rat glomerular mesangial cells and is augmented in immune complex glomerulonephritis. J Immunol. 1987 May 15;138(10):3207–3212. [PubMed] [Google Scholar]
- Yamamoto T., Wilson C. B. Complement dependence of antibody-induced mesangial cell injury in the rat. J Immunol. 1987 Jun 1;138(11):3758–3765. [PubMed] [Google Scholar]
- Yoshioka K., Takemura T., Murakami K., Okada M., Yagi K., Miyazato H., Matsushima K., Maki S. In situ expression of cytokines in IgA nephritis. Kidney Int. 1993 Oct;44(4):825–833. doi: 10.1038/ki.1993.317. [DOI] [PubMed] [Google Scholar]
- Young B. A., Johnson R. J., Alpers C. E., Eng E., Gordon K., Floege J., Couser W. G., Seidel K. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995 Mar;47(3):935–944. doi: 10.1038/ki.1995.139. [DOI] [PubMed] [Google Scholar]
- van Goor H., van der Horst M. L., Fidler V., Grond J. Glomerular macrophage modulation affects mesangial expansion in the rat after renal ablation. Lab Invest. 1992 May;66(5):564–571. [PubMed] [Google Scholar]