Abstract
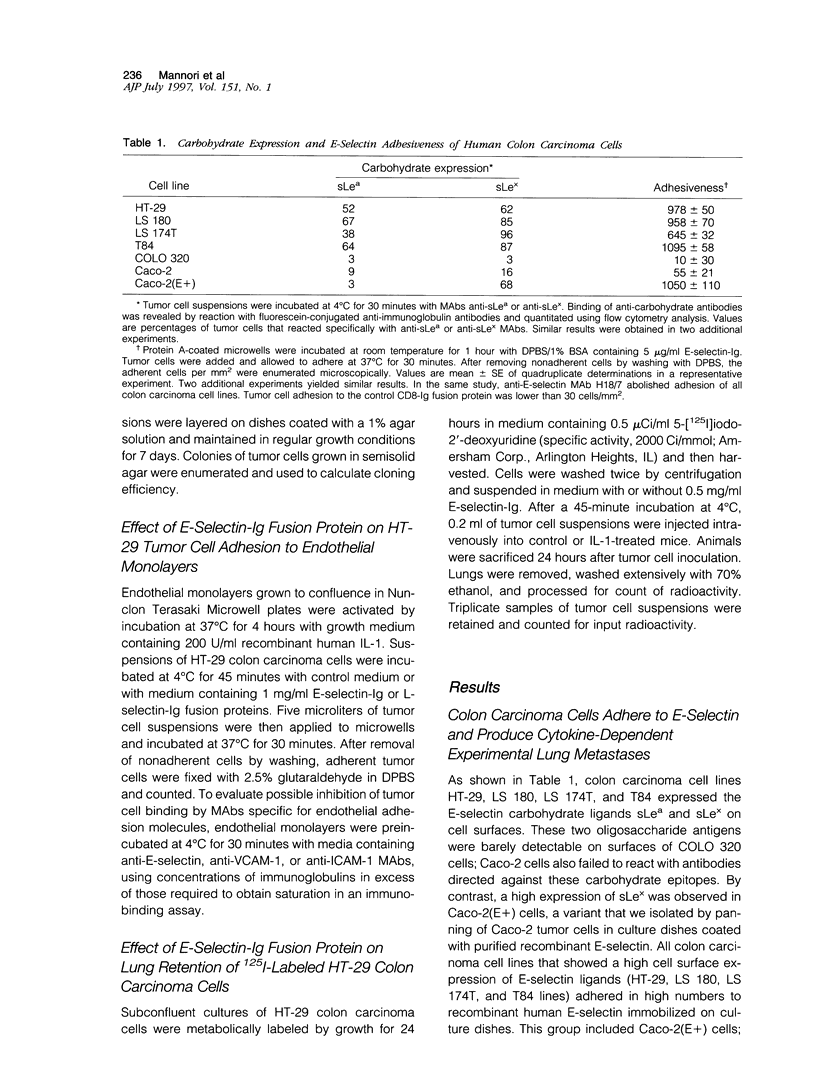
During metastasis, tumor cells adhere to vascular endothelia. E-selectin is an adhesive protein expressed by cytokine-activated endothelium that can support adhesion of colon cancer cells through the recognition of specific carbohydrate ligands. Using a series of colon carcinoma cell lines that displayed E-selectin adhesiveness and an increased metastatic capacity in cytokine-treated mice, we examined possible inhibition of cytokine-dependent experimental lung metastasis by a soluble form of E-selectin, the recombinant fusion protein E-selectin-immunoglobulin. We found that E-selectin-immunoglobulin bound to the surfaces of HT-29 colon carcinoma cells and blocked the formation of cytokine-inducible experimental lung metastases; control L-selectin-immunoglobulin also bound to HT-29 cells but had no effect on tumor cell lung colonization. E-selectin-immunoglobulin was found to interfere with E-selectin-dependent adhesion of HT-29 cells to activated vascular endothelium and to block the retention of these cells in the lung, a process that implies tumor cell adhesive interactions with the host vasculature. Our results demonstrate that E-selectin-immunoglobulin inhibits adhesion and formation of lung metastases by colon carcinoma cells and suggest that impairment of tumor cell-endothelium adhesion might represent a therapeutic approach to the metastatic diffusion of tumors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arguello F., Baggs R. B., Graves B. T., Harwell S. E., Cohen H. J., Frantz C. N. Effect of IL-1 on experimental bone/bone-marrow metastases. Int J Cancer. 1992 Nov 11;52(5):802–807. doi: 10.1002/ijc.2910520522. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Kolanus W., Walz G., Fredman P., Seed B. CD62/P-selectin recognition of myeloid and tumor cell sulfatides. Cell. 1991 Oct 4;67(1):35–44. doi: 10.1016/0092-8674(91)90570-o. [DOI] [PubMed] [Google Scholar]
- Bani M. R., Garofalo A., Scanziani E., Giavazzi R. Effect of interleukin-1-beta on metastasis formation in different tumor systems. J Natl Cancer Inst. 1991 Jan 16;83(2):119–123. doi: 10.1093/jnci/83.2.119. [DOI] [PubMed] [Google Scholar]
- Becker-André M., Hooft van Huijsduijnen R., Losberger C., Whelan J., Delamarter J. F. Murine endothelial leukocyte-adhesion molecule 1 is a close structural and functional homologue of the human protein. Eur J Biochem. 1992 Jun 1;206(2):401–411. doi: 10.1111/j.1432-1033.1992.tb16940.x. [DOI] [PubMed] [Google Scholar]
- Bennicelli J. L., Elias J., Kern J., Guerry D., 4th Production of interleukin 1 activity by cultured human melanoma cells. Cancer Res. 1989 Feb 15;49(4):930–935. [PubMed] [Google Scholar]
- Berg E. L., Magnani J., Warnock R. A., Robinson M. K., Butcher E. C. Comparison of L-selectin and E-selectin ligand specificities: the L-selectin can bind the E-selectin ligands sialyl Le(x) and sialyl Le(a). Biochem Biophys Res Commun. 1992 Apr 30;184(2):1048–1055. doi: 10.1016/0006-291x(92)90697-j. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Mansson O., Butcher E. C., Magnani J. L. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991 Aug 15;266(23):14869–14872. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M., Mannori G., Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994;45:361–378. doi: 10.1146/annurev.med.45.1.361. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancone L., Araki M., Araki K., Vassalli P., Stamenkovic I. Redirection of tumor metastasis by expression of E-selectin in vivo. J Exp Med. 1996 Feb 1;183(2):581–587. doi: 10.1084/jem.183.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows F. J., Haskard D. O., Hart I. R., Marshall J. F., Selkirk S., Poole S., Thorpe P. E. Influence of tumor-derived interleukin 1 on melanoma-endothelial cell interactions in vitro. Cancer Res. 1991 Sep 15;51(18):4768–4775. [PubMed] [Google Scholar]
- Dejana E., Martin-Padura I., Lauri D., Bernasconi S., Bani M. R., Garofalo A., Giavazzi R., Magnani J., Mantovani A., Menard S. Endothelial leukocyte adhesion molecule-1-dependent adhesion of colon carcinoma cells to vascular endothelium is inhibited by an antibody to Lewis fucosylated type I carbohydrate chain. Lab Invest. 1992 Mar;66(3):324–330. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Foxall C., Watson S. R., Dowbenko D., Fennie C., Lasky L. A., Kiso M., Hasegawa A., Asa D., Brandley B. K. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol. 1992 May;117(4):895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Hirota M., Terasaki P. I., Wakisaka A., Togashi H., Chia D., Suyama N., Fukushi Y., Nudelman E., Hakomori S. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Res. 1984 Nov;44(11):5279–5285. [PubMed] [Google Scholar]
- Giavazzi R., Foppolo M., Dossi R., Remuzzi A. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest. 1993 Dec;92(6):3038–3044. doi: 10.1172/JCI116928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavazzi R., Garofalo A., Bani M. R., Abbate M., Ghezzi P., Boraschi D., Mantovani A., Dejana E. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990 Aug 1;50(15):4771–4775. [PubMed] [Google Scholar]
- Gundel R. H., Wegner C. D., Torcellini C. A., Clarke C. C., Haynes N., Rothlein R., Smith C. W., Letts L. G. Endothelial leukocyte adhesion molecule-1 mediates antigen-induced acute airway inflammation and late-phase airway obstruction in monkeys. J Clin Invest. 1991 Oct;88(4):1407–1411. doi: 10.1172/JCI115447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa K., Nudelman E. D., Stroud M. R., Shiozawa T., Hakomori S. Selectin GMP-140 (CD62; PADGEM) binds to sialosyl-Le(a) and sialosyl-Le(x), and sulfated glycans modulate this binding. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1223–1230. doi: 10.1016/0006-291x(91)92069-v. [DOI] [PubMed] [Google Scholar]
- Hoff S. D., Irimura T., Matsushita Y., Ota D. M., Cleary K. R., Hakomori S. Metastatic potential of colon carcinoma. Expression of ABO/Lewis-related antigens. Arch Surg. 1990 Feb;125(2):206–209. doi: 10.1001/archsurg.1990.01410140084013. [DOI] [PubMed] [Google Scholar]
- Honn K. V., Tang D. G. Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992 Nov;11(3-4):353–375. doi: 10.1007/BF01307187. [DOI] [PubMed] [Google Scholar]
- Honn K. V., Tang D. G., Chen Y. Q. Platelets and cancer metastasis: more than an epiphenomenon. Semin Thromb Hemost. 1992;18(4):392–415. doi: 10.1055/s-2007-1002578. [DOI] [PubMed] [Google Scholar]
- Itzkowitz S. H., Yuan M., Fukushi Y., Palekar A., Phelps P. C., Shamsuddin A. M., Trump B. F., Hakomori S., Kim Y. S. Lewisx- and sialylated Lewisx-related antigen expression in human malignant and nonmalignant colonic tissues. Cancer Res. 1986 May;46(5):2627–2632. [PubMed] [Google Scholar]
- Kishimoto T., Ishikura H., Kimura C., Takahashi T., Kato H., Yoshiki T. Phenotypes correlating to metastatic properties of pancreas adenocarcinoma in vivo: the importance of surface sialyl Lewis(a) antigen. Int J Cancer. 1996 Aug 22;69(4):290–294. doi: 10.1002/(SICI)1097-0215(19960822)69:4<290::AID-IJC9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kojima N., Handa K., Newman W., Hakomori S. Inhibition of selectin-dependent tumor cell adhesion to endothelial cells and platelets by blocking O-glycosylation of these cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1288–1295. doi: 10.1016/0006-291x(92)91872-n. [DOI] [PubMed] [Google Scholar]
- Köck A., Schwarz T., Urbanski A., Peng Z., Vetterlein M., Micksche M., Ansel J. C., Kung H. F., Luger T. A. Expression and release of interleukin-1 by different human melanoma cell lines. J Natl Cancer Inst. 1989 Jan 4;81(1):36–42. doi: 10.1093/jnci/81.1.36. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Lauri D., Needham L., Martin-Padura I., Dejana E. Tumor cell adhesion to endothelial cells: endothelial leukocyte adhesion molecule-1 as an inducible adhesive receptor specific for colon carcinoma cells. J Natl Cancer Inst. 1991 Sep 18;83(18):1321–1324. doi: 10.1093/jnci/83.18.1321. [DOI] [PubMed] [Google Scholar]
- Lee W. P., Gribling P., De Guzman L., Ehsani N., Watson S. R. A P-selectin-immunoglobulin G chimera is protective in a rabbit ear model of ischemia-reperfusion. Surgery. 1995 Apr;117(4):458–465. doi: 10.1016/s0039-6060(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Ley K., Gaehtgens P., Fennie C., Singer M. S., Lasky L. A., Rosen S. D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991 Jun 15;77(12):2553–2555. [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Mannori G., Cecconi O., Mugnai G., Ruggieri S. Role of glycolipids in the metastatic process: characteristics of neutral glycolipids in clones with different metastatic potentials isolated from a murine fibrosarcoma cell line. Int J Cancer. 1990 May 15;45(5):984–988. doi: 10.1002/ijc.2910450535. [DOI] [PubMed] [Google Scholar]
- Mannori G., Crottet P., Cecconi O., Hanasaki K., Aruffo A., Nelson R. M., Varki A., Bevilacqua M. P. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res. 1995 Oct 1;55(19):4425–4431. [PubMed] [Google Scholar]
- Mannori G., Mugnai G., Ruggieri S. Lectin reactivity of murine fibrosarcoma lines with a different metastatic potential. Cancer Lett. 1991 Aug;59(2):133–138. doi: 10.1016/0304-3835(91)90177-j. [DOI] [PubMed] [Google Scholar]
- Martens C. L., Cwirla S. E., Lee R. Y., Whitehorn E., Chen E. Y., Bakker A., Martin E. L., Wagstrom C., Gopalan P., Smith C. W. Peptides which bind to E-selectin and block neutrophil adhesion. J Biol Chem. 1995 Sep 8;270(36):21129–21136. doi: 10.1074/jbc.270.36.21129. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Selectins. Curr Opin Immunol. 1994 Feb;6(1):75–84. doi: 10.1016/0952-7915(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Varani J., Dame M. K., Lane C. L., Smith C. W., Anderson D. C., Ward P. A. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991 Oct;88(4):1396–1406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Watson S. R., Fennie C., Ward P. A. Protective effects of selectin chimeras in neutrophil-mediated lung injury. J Immunol. 1993 Dec 1;151(11):6410–6417. [PubMed] [Google Scholar]
- Nelson R. M., Aruffo A., Dolich S., Cecconi O., Mannori G., Bevilacqua M. P. Quantitative determination of selectin-carbohydrate interactions. Cold Spring Harb Symp Quant Biol. 1992;57:271–279. doi: 10.1101/sqb.1992.057.01.032. [DOI] [PubMed] [Google Scholar]
- Nelson R. M., Dolich S., Aruffo A., Cecconi O., Bevilacqua M. P. Higher-affinity oligosaccharide ligands for E-selectin. J Clin Invest. 1993 Mar;91(3):1157–1166. doi: 10.1172/JCI116275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Orosz P., Echtenacher B., Falk W., Rüschoff J., Weber D., Männel D. N. Enhancement of experimental metastasis by tumor necrosis factor. J Exp Med. 1993 May 1;177(5):1391–1398. doi: 10.1084/jem.177.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Behringer R. R., Quaife C. J., Maxwell F., Maxwell I. H., Brinster R. L. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987 Jul 31;50(3):435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Pauli B. U., Augustin-Voss H. G., el-Sabban M. E., Johnson R. C., Hammer D. A. Organ-preference of metastasis. The role of endothelial cell adhesion molecules. Cancer Metastasis Rev. 1990 Nov;9(3):175–189. doi: 10.1007/BF00046359. [DOI] [PubMed] [Google Scholar]
- Pilewski J. M., Yan H. C., Juhasz I., Christofidou-Solomidou M., Williams J., Murphy G. F., Albelda S. M. Modulation of adhesion molecules by cytokines in vivo using human/severe combined immunodeficient (SCID) mouse chimeras. J Clin Immunol. 1995 Nov;15(6 Suppl):122S–129S. doi: 10.1007/BF01540902. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Polley M. J., Phillips M. L., Wayner E., Nudelman E., Singhal A. K., Hakomori S., Paulson J. C. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. D., Bertozzi C. R. The selectins and their ligands. Curr Opin Cell Biol. 1994 Oct;6(5):663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Saitoh O., Wang W. C., Lotan R., Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992 Mar 15;267(8):5700–5711. [PubMed] [Google Scholar]
- Sawada R., Lowe J. B., Fukuda M. E-selectin-dependent adhesion efficiency of colonic carcinoma cells is increased by genetic manipulation of their cell surface lysosomal membrane glycoprotein-1 expression levels. J Biol Chem. 1993 Jun 15;268(17):12675–12681. [PubMed] [Google Scholar]
- Sawada R., Tsuboi S., Fukuda M. Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1994 Jan 14;269(2):1425–1431. [PubMed] [Google Scholar]
- Spriggs D., Imamura K., Rodriguez C., Horiguchi J., Kufe D. W. Induction of tumor necrosis factor expression and resistance in a human breast tumor cell line. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6563–6566. doi: 10.1073/pnas.84.18.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. P., Wagner D. D. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest. 1993 Aug;92(2):804–813. doi: 10.1172/JCI116654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A., Ohmori K., Yoneda T., Tsuyuoka K., Hasegawa A., Kiso M., Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993 Jan 15;53(2):354–361. [PubMed] [Google Scholar]
- Tyrrell D., James P., Rao N., Foxall C., Abbas S., Dasgupta F., Nashed M., Hasegawa A., Kiso M., Asa D. Structural requirements for the carbohydrate ligand of E-selectin. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10372–10376. doi: 10.1073/pnas.88.22.10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözeren A., Kleinman H. K., Grant D. S., Morales D., Mercurio A. M., Byers S. W. E-selectin-mediated dynamic interactions of breast- and colon-cancer cells with endothelial-cell monolayers. Int J Cancer. 1995 Jan 27;60(3):426–431. doi: 10.1002/ijc.2910600326. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F., Alvarez A., Asumendi A., Urcelay B., Tonino P., Dinarello C. A. Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst. 1996 Feb 21;88(3-4):198–205. doi: 10.1093/jnci/88.3-4.198. [DOI] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Fennie C., Lasky L. A. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature. 1991 Jan 10;349(6305):164–167. doi: 10.1038/349164a0. [DOI] [PubMed] [Google Scholar]
- Watson S. R. L-selectin-IgG chimera--in vitro and in vivo. Agents Actions Suppl. 1993;41:103–109. [PubMed] [Google Scholar]
- Weiss L., Grundmann E., Torhorst J., Hartveit F., Moberg I., Eder M., Fenoglio-Preiser C. M., Napier J., Horne C. H., Lopez M. J. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986 Nov;150(3):195–203. doi: 10.1002/path.1711500308. [DOI] [PubMed] [Google Scholar]
- Weiss L., Orr F. W., Honn K. V. Interactions between cancer cells and the microvasculature: a rate-regulator for metastasis. Clin Exp Metastasis. 1989 Mar-Apr;7(2):127–167. doi: 10.1007/BF01787020. [DOI] [PubMed] [Google Scholar]
- Whitworth P. W., Pak C. C., Esgro J., Kleinerman E. S., Fidler I. J. Macrophages and cancer. Cancer Metastasis Rev. 1990 Feb;8(4):319–351. doi: 10.1007/BF00052607. [DOI] [PubMed] [Google Scholar]
- Zetter B. R. The cellular basis of site-specific tumor metastasis. N Engl J Med. 1990 Mar 1;322(9):605–612. doi: 10.1056/NEJM199003013220907. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Moore K. L., Smith D. F., Varki A., McEver R. P., Cummings R. D. The selectin GMP-140 binds to sialylated, fucosylated lactosaminoglycans on both myeloid and nonmyeloid cells. J Cell Biol. 1991 Oct;115(2):557–564. doi: 10.1083/jcb.115.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]


