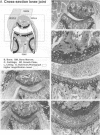
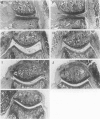
Abstract
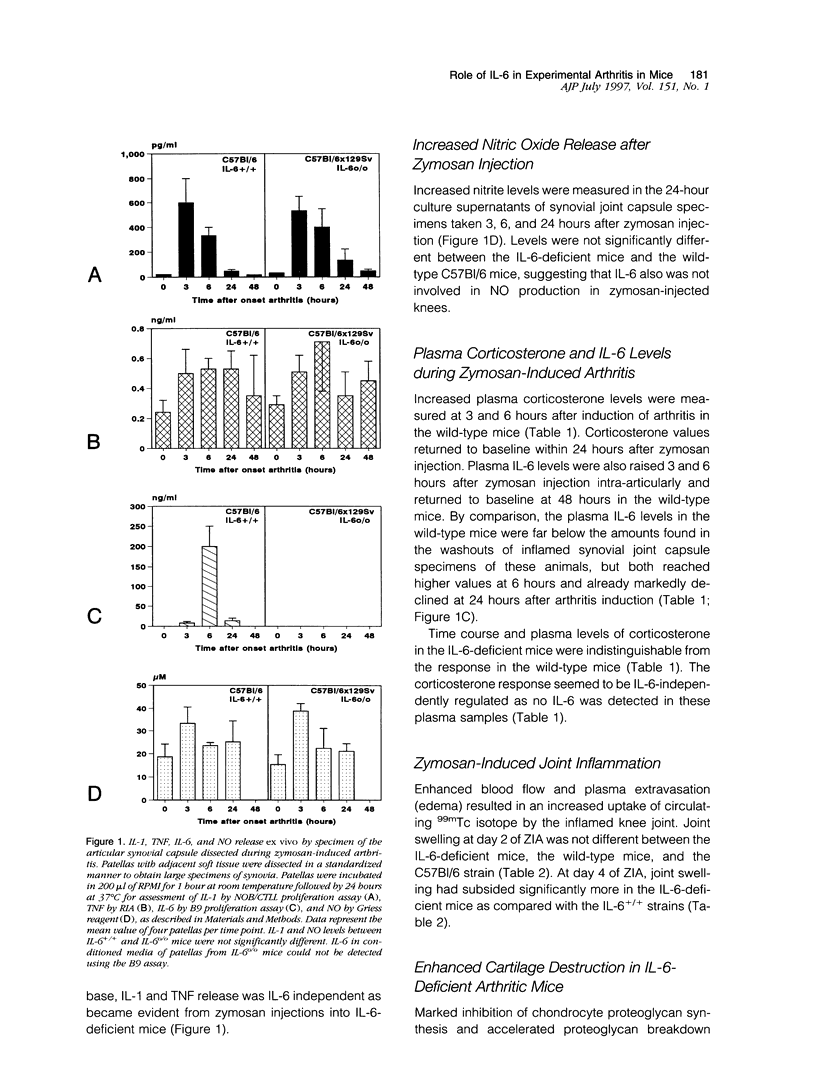
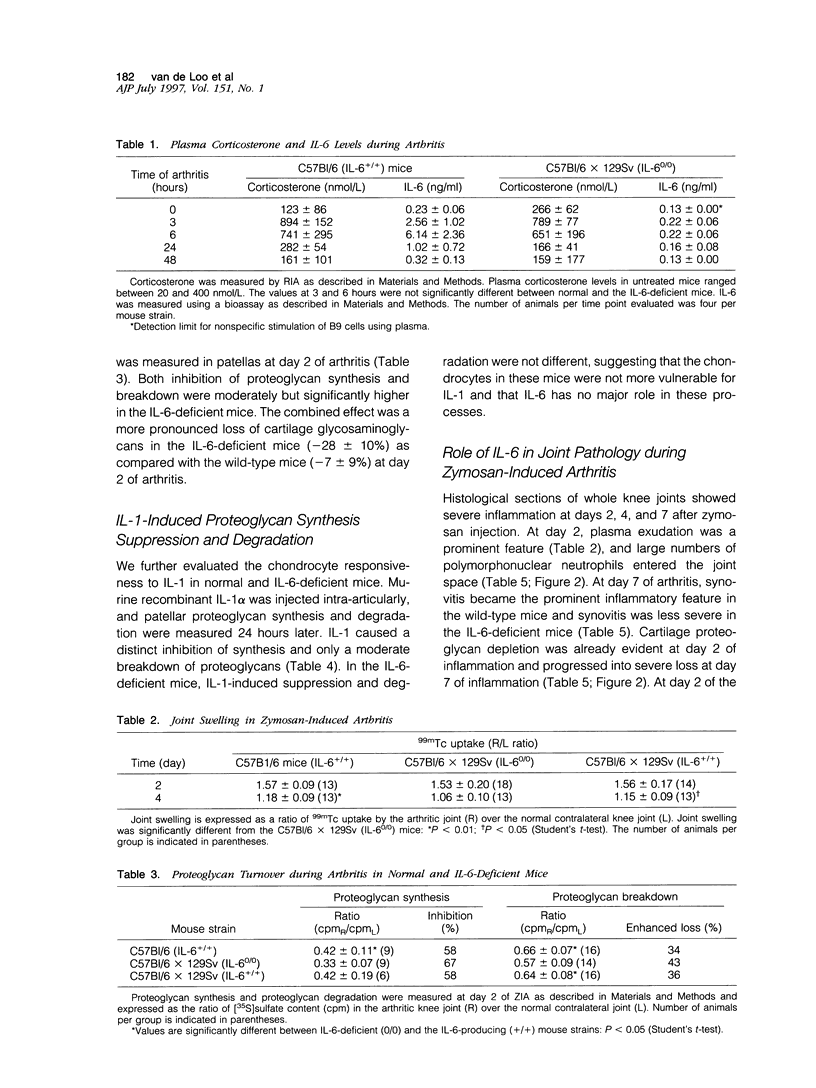
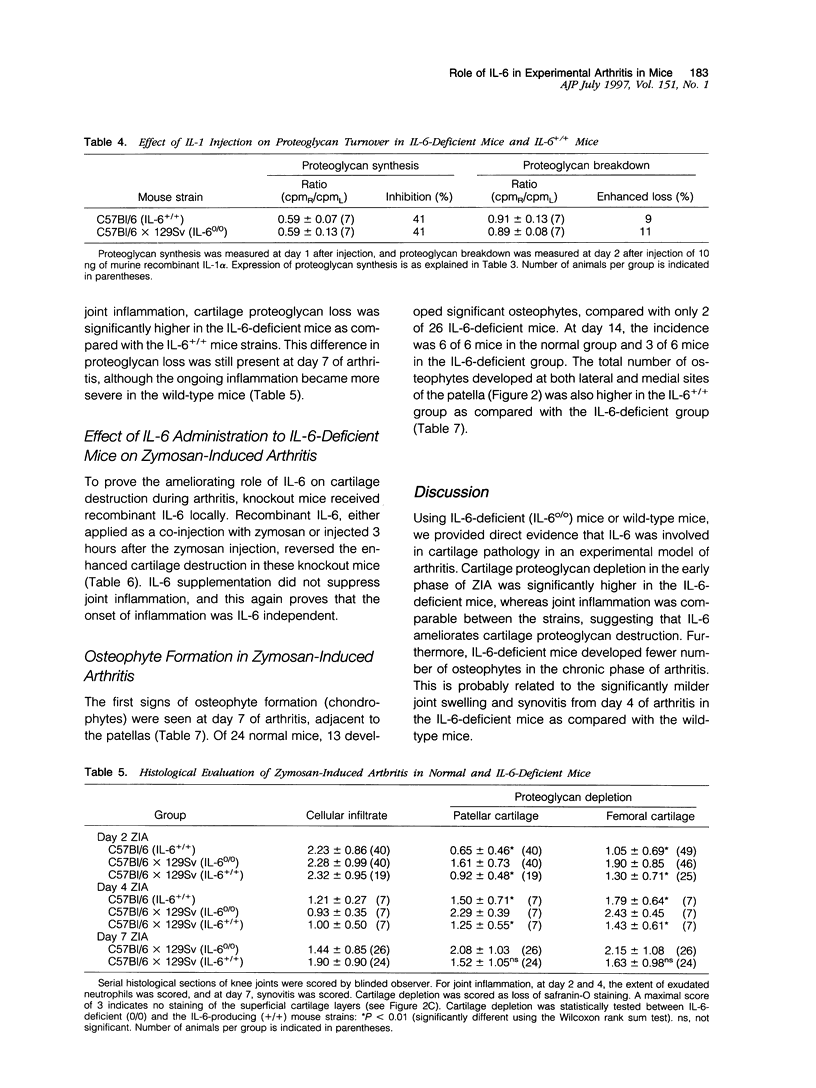
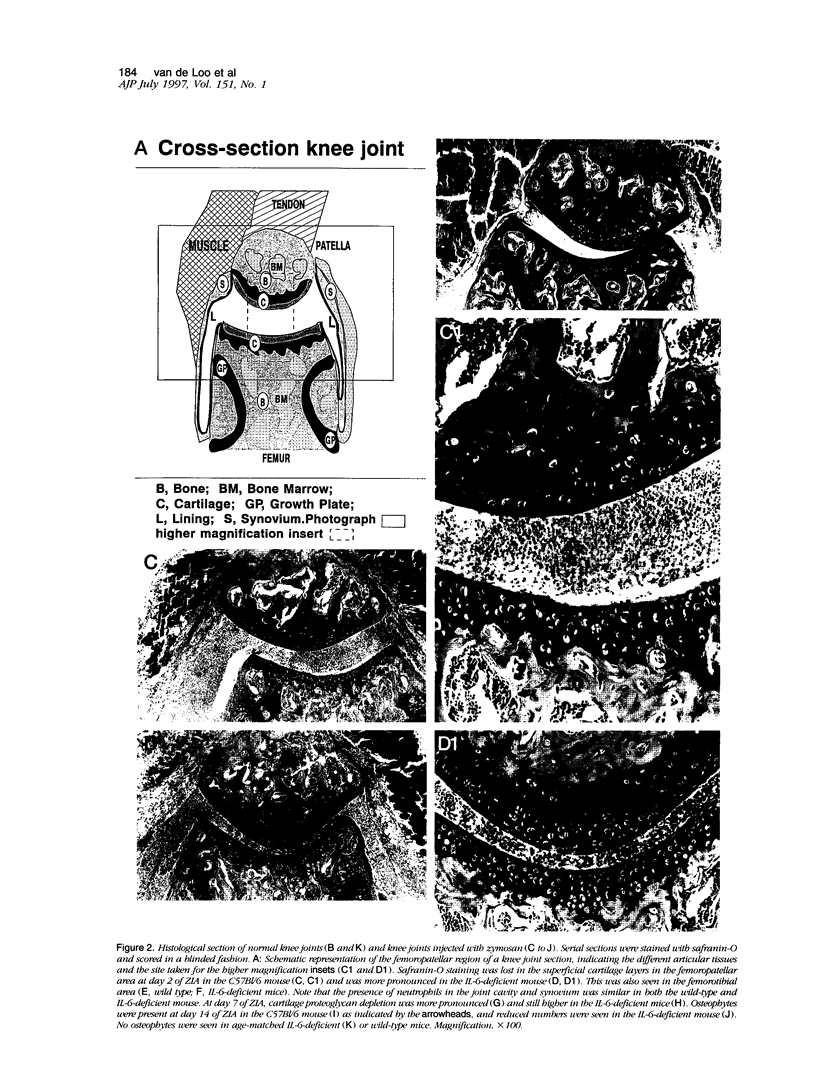
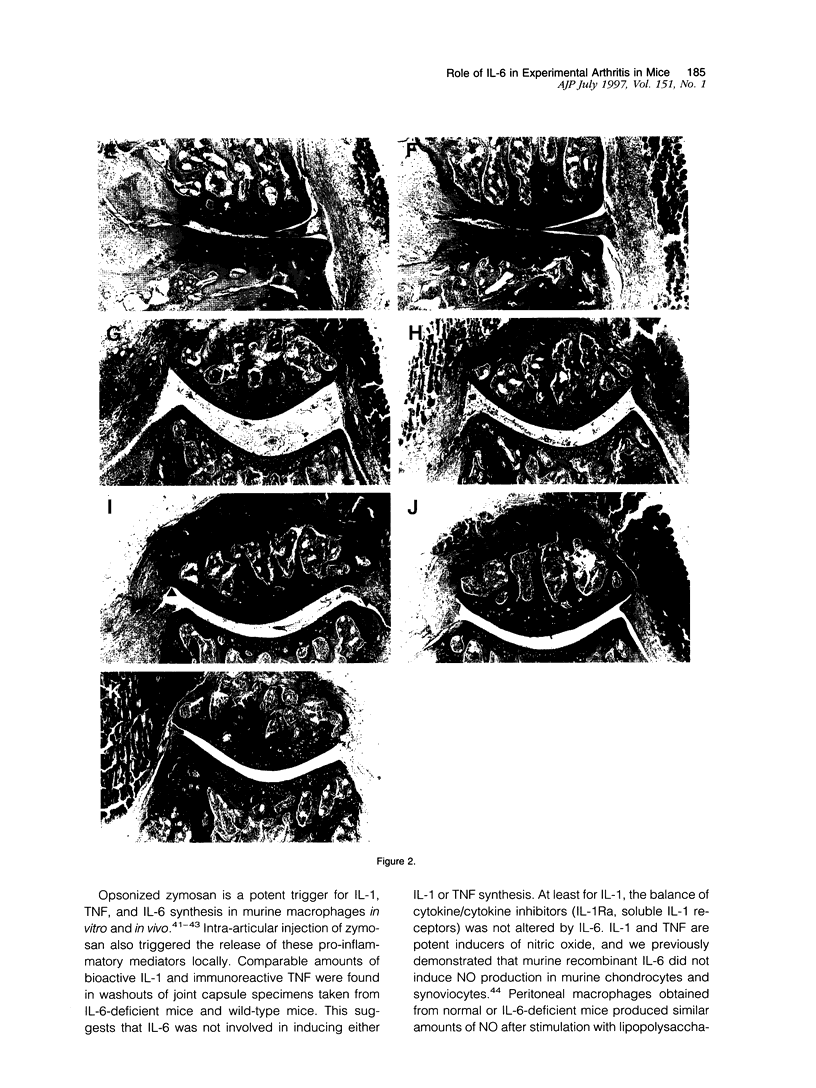
Using interleukin (IL)-6-deficient (IL-6(0/0) mice or wild-type mice, we investigated the controversial role of IL-6 in joint inflammation and cartilage pathology during zymosan-induced arthritis (ZIA). Monoarticular arthritis was elicited by injection of zymosan into the right knee joint cavity. Production of IL-1, tumor necrosis factor (TNF), IL-6, and nitric oxide by the inflamed knee was assessed in washouts of joint capsule specimens. Plasma corticosterone was measured using a radioimmunoassay. Proteoglycan synthesis was assessed using [35S]sulfate incorporation into patellas ex vivo. Joint swelling was quantified by joint uptake of circulating 99mTechnetium pertechnetate. Histology was taken to evaluate cellular infiltration and cartilage damage. Zymosan caused a rapid increase in articular IL-1, IL-6, TNF, and NO levels. Except for IL-6, the released amounts and time course of these mediators were comparable in the IL-6-deficient mice and the wild-type mice. Elevated plasma corticosterone levels were measured during the first day of arthritis in both strains. At day 2 of ZIA, joint inflammation (joint swelling and cell exudate) in IL-6-deficient mice was comparable with that in the wild-type mice. The marked suppression of chondrocyte proteoglycan synthesis and proteoglycan degradation were on the average higher in the IL-6-deficient mice. Together this resulted in a more pronounced proteoglycan depletion in the IL-6-deficient mice as compared with the wild-type mice during the first week of arthritis. Injection of recombinant IL-6 into the joint cavity corrected the IL-6 deficiency and significantly reduced cartilage destruction. Inflammation was more chronic in the wild-type mice, and these mice also showed a higher prevalence for osteophyte formation. In ZIA, IL-6 plays a dual role in connective tissue pathology, reducing proteoglycan loss in the acute phase and enhancing osteophyte formation in the chronic phase. The latter could be related to the more severe joint inflammation as seen in the normal (IL-6-producing) animals during the chronic phase of arthritis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Barton B. E. The biological effects of interleukin 6. Med Res Rev. 1996 Jan;16(1):87–109. doi: 10.1002/(SICI)1098-1128(199601)16:1<87::AID-MED3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol Today. 1994 Feb;15(2):74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Bender S., Haubeck H. D., Van de Leur E., Dufhues G., Schiel X., Lauwerijns J., Greiling H., Heinrich P. C. Interleukin-1 beta induces synthesis and secretion of interleukin-6 in human chondrocytes. FEBS Lett. 1990 Apr 24;263(2):321–324. doi: 10.1016/0014-5793(90)81404-c. [DOI] [PubMed] [Google Scholar]
- Biffl W. L., Moore E. E., Moore F. A., Barnett C. C., Jr Interleukin-6 suppression of neutrophil apoptosis is neutrophil concentration dependent. J Leukoc Biol. 1995 Nov;58(5):582–584. doi: 10.1002/jlb.58.5.582. [DOI] [PubMed] [Google Scholar]
- Bluethmann H., Rothe J., Schultze N., Tkachuk M., Koebel P. Establishment of the role of IL-6 and TNF receptor 1 using gene knockout mice. J Leukoc Biol. 1994 Nov;56(5):565–570. doi: 10.1002/jlb.56.5.565. [DOI] [PubMed] [Google Scholar]
- Bromander A. K., Ekman L., Kopf M., Nedrud J. G., Lycke N. Y. IL-6-deficient mice exhibit normal mucosal IgA responses to local immunizations and Helicobacter felis infection. J Immunol. 1996 Jun 1;156(11):4290–4297. [PubMed] [Google Scholar]
- Brozik M., Rosztóczy I., Merétey K., Bálint G., Gaál M., Balogh Z., Bart M., Mituszova M., Velics V., Falus A. Interleukin 6 levels in synovial fluids of patients with different arthritides: correlation with local IgM rheumatoid factor and systemic acute phase protein production. J Rheumatol. 1992 Jan;19(1):63–68. [PubMed] [Google Scholar]
- Chai Z., Gatti S., Toniatti C., Poli V., Bartfai T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 beta: a study on IL-6-deficient mice. J Exp Med. 1996 Jan 1;183(1):311–316. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J., Rordorf-Adam C., Geiger T., Towbin H., Kunz S., Nguyen H., Zingel O., Chaplin D., Vosbeck K. Interleukin-1 (IL-1) production in a mouse tissue chamber model of inflammation. I. Development and initial characterisation of the model. Agents Actions. 1993 Mar;38(3-4):247–254. doi: 10.1007/BF01976217. [DOI] [PubMed] [Google Scholar]
- Drenth J. P., Van Uum S. H., Van Deuren M., Pesman G. J., Van der Ven-Jongekrijg J., Van der Meer J. W. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. J Appl Physiol (1985) 1995 Nov;79(5):1497–1503. doi: 10.1152/jappl.1995.79.5.1497. [DOI] [PubMed] [Google Scholar]
- Erdö F., Török K., Székely J. I. Measurement of interleukin-1 liberation in zymosan air-pouch exudate in mice. Agents Actions. 1994 Mar;41(1-2):93–95. doi: 10.1007/BF01986403. [DOI] [PubMed] [Google Scholar]
- Fattori E., Cappelletti M., Costa P., Sellitto C., Cantoni L., Carelli M., Faggioni R., Fantuzzi G., Ghezzi P., Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994 Oct 1;180(4):1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechtenmacher J., Huch K., Thonar E. J., Mollenhauer J. A., Davies S. R., Schmid T. M., Puhl W., Sampath T. K., Aydelotte M. B., Kuettner K. E. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996 Nov;39(11):1896–1904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing A. J., Bird C. R., Bristow A., Poole S., Thorpe R. A simple sensitive bioassay for interleukin-1 which is unresponsive to 10(3) U/ml of interleukin-2. J Immunol Methods. 1987 May 4;99(1):7–11. doi: 10.1016/0022-1759(87)90025-1. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Carson D. A., Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990 Jan 15;144(2):499–505. [PubMed] [Google Scholar]
- Guerne P. A., Zuraw B. L., Vaughan J. H., Carson D. A., Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989 Feb;83(2):585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann E., Fleischer B., Mayet W. J., Poralla T., Meyer zum Büschenfelde K. H. Correlation of synovial fluid interleukin 6 (IL-6) activities with IgG concentrations in patients with inflammatory joint disease and osteoarthritis. Clin Exp Rheumatol. 1989 Jul-Aug;7(4):411–414. [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Holt I., Cooper R. G., Hopkins S. J. Relationships between local inflammation, interleukin-6 concentration and the acute phase protein response in arthritis patients. Eur J Clin Invest. 1991 Oct;21(5):479–484. doi: 10.1111/j.1365-2362.1991.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Ito A., Itoh Y., Sasaguri Y., Morimatsu M., Mori Y. Effects of interleukin-6 on the metabolism of connective tissue components in rheumatoid synovial fibroblasts. Arthritis Rheum. 1992 Oct;35(10):1197–1201. doi: 10.1002/art.1780351012. [DOI] [PubMed] [Google Scholar]
- Joosten L. A., Helsen M. M., van de Loo F. A., van den Berg W. B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996 May;39(5):797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Schorlemmer H. U., Pope C., Allison A. C. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 1977 Sep-Oct;20(7):1396–1401. doi: 10.1002/art.1780200714. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Akira S., Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992 Oct 23;258(5082):593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- Kopf M., Baumann H., Freer G., Freudenberg M., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Köhler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994 Mar 24;368(6469):339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Libert C., Takahashi N., Cauwels A., Brouckaert P., Bluethmann H., Fiers W. Response of interleukin-6-deficient mice to tumor necrosis factor-induced metabolic changes and lethality. Eur J Immunol. 1994 Sep;24(9):2237–2242. doi: 10.1002/eji.1830240945. [DOI] [PubMed] [Google Scholar]
- Lotz M. Interleukin-6. Cancer Invest. 1993;11(6):732–742. doi: 10.3109/07357909309046948. [DOI] [PubMed] [Google Scholar]
- Luyten F. P., Chen P., Paralkar V., Reddi A. H. Recombinant bone morphogenetic protein-4, transforming growth factor-beta 1, and activin A enhance the cartilage phenotype of articular chondrocytes in vitro. Exp Cell Res. 1994 Feb;210(2):224–229. doi: 10.1006/excr.1994.1033. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Allen J. B., Mizel D. E., Albina J. E., Xie Q. W., Nathan C. F., Wahl S. M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993 Aug 1;178(2):749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto O., Yamada H., Mukaida M., Shimmei M. Stimulation of TIMP-1 production by oncostatin M in human articular cartilage. Arthritis Rheum. 1996 Apr;39(4):560–566. doi: 10.1002/art.1780390404. [DOI] [PubMed] [Google Scholar]
- Perretti M., Solito E., Parente L. Evidence that endogenous interleukin-1 is involved in leukocyte migration in acute experimental inflammation in rats and mice. Agents Actions. 1992 Jan;35(1-2):71–78. doi: 10.1007/BF01990954. [DOI] [PubMed] [Google Scholar]
- Poli V., Balena R., Fattori E., Markatos A., Yamamoto M., Tanaka H., Ciliberto G., Rodan G. A., Costantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994 Mar 1;13(5):1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay A. J., Husband A. J., Ramshaw I. A., Bao S., Matthaei K. I., Koehler G., Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994 Apr 22;264(5158):561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- Riedy M. C., Stewart C. C. Inhibitory role of interleukin-6 in macrophage proliferation. J Leukoc Biol. 1992 Jul;52(1):125–127. doi: 10.1002/jlb.52.1.125. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. T., Cugnini R., Tara D. C., Hefeneider S., Ansel J. C. Production and modulation of interleukin 6 synthesis by synoviocytes derived from patients with arthritic disease. Ann Rheum Dis. 1992 Feb;51(2):198–202. doi: 10.1136/ard.51.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk J., van den Berg W. B., van de Putte L. B., Joosten L. A., van der Sluis M. Effects of experimental joint inflammation on bone marrow and periarticular bone. A study of two types of arthritis, using variable degrees of inflammation. Br J Exp Pathol. 1985 Aug;66(4):435–444. [PMC free article] [PubMed] [Google Scholar]
- Shingu M., Isayama T., Yasutake C., Naono T., Nobunaga M., Tomari K., Horie K., Goto Y. Role of oxygen radicals and IL-6 in IL-1-dependent cartilage matrix degradation. Inflammation. 1994 Dec;18(6):613–623. doi: 10.1007/BF01535259. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Meyers K., Meschter C., Coffey J. W., Hoffman R. A., Evans C. H. N-monomethyl arginine, an inhibitor of nitric oxide synthase, suppresses the development of adjuvant arthritis in rats. Arthritis Rheum. 1994 Jul;37(7):1062–1069. doi: 10.1002/art.1780370712. [DOI] [PubMed] [Google Scholar]
- Sweep C. G., van der Meer M. J., Hermus A. R., Smals A. G., van der Meer J. W., Pesman G. J., Willemsen S. J., Benraad T. J., Kloppenborg P. W. Chronic stimulation of the pituitary-adrenal axis in rats by interleukin-1 beta infusion: in vivo and in vitro studies. Endocrinology. 1992 Mar;130(3):1153–1164. doi: 10.1210/endo.130.3.1311230. [DOI] [PubMed] [Google Scholar]
- Tilg H., Trehu E., Atkins M. B., Dinarello C. A., Mier J. W. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994 Jan 1;83(1):113–118. [PubMed] [Google Scholar]
- Tyler J. A. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989 Jun 1;260(2):543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure P. J., Joosten L. A., van der Kraan P. M., Van den Berg W. B. Responsiveness of articular cartilage from normal and inflamed mouse knee joints to various growth factors. Ann Rheum Dis. 1994 Jul;53(7):455–460. doi: 10.1136/ard.53.7.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger P. M., Kusari A. B., ten Dijke P., Lotz M. IL-1 beta and IL-6 selectively induce transforming growth factor-beta isoforms in human articular chondrocytes. J Immunol. 1993 Sep 15;151(6):3337–3344. [PubMed] [Google Scholar]
- Vladutiu A. O. Role of nitric oxide in autoimmunity. Clin Immunol Immunopathol. 1995 Jul;76(1 Pt 1):1–11. doi: 10.1006/clin.1995.1081. [DOI] [PubMed] [Google Scholar]
- Waage A., Kaufmann C., Espevik T., Husby G. Interleukin-6 in synovial fluid from patients with arthritis. Clin Immunol Immunopathol. 1989 Mar;50(3):394–398. doi: 10.1016/0090-1229(89)90146-3. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Granger D. L., Pisetsky D. S., Seldin M. F., Misukonis M. A., Mason S. N., Pippen A. M., Ruiz P., Wood E. R., Gilkeson G. S. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994 Feb 1;179(2):651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolvekamp M. C., Marquet R. L. Interleukin-6: historical background, genetics and biological significance. Immunol Lett. 1990 Mar-Apr;24(1):1–9. doi: 10.1016/0165-2478(90)90028-o. [DOI] [PubMed] [Google Scholar]
- Wood N. C., Symons J. A., Dickens E., Duff G. W. In situ hybridization of IL-6 in rheumatoid arthritis. Clin Exp Immunol. 1992 Feb;87(2):183–189. doi: 10.1111/j.1365-2249.1992.tb02972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley P. H., Dutcher J., Widmer M. B., Gillis S. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J Immunol. 1993 Dec 1;151(11):6602–6607. [PubMed] [Google Scholar]
- Wooley P. H., Whalen J. D., Chapman D. L., Berger A. E., Richard K. A., Aspar D. G., Staite N. D. The effect of an interleukin-1 receptor antagonist protein on type II collagen-induced arthritis and antigen-induced arthritis in mice. Arthritis Rheum. 1993 Sep;36(9):1305–1314. doi: 10.1002/art.1780360915. [DOI] [PubMed] [Google Scholar]
- Yeh L. A., Augustine A. J., Lee P., Riviere L. R., Sheldon A. Interleukin-4, an inhibitor of cartilage breakdown in bovine articular cartilage explants. J Rheumatol. 1995 Sep;22(9):1740–1746. [PubMed] [Google Scholar]
- Zhou D. H., Munster A. M., Winchurch R. A. Inhibitory effects of interleukin 6 on immunity. Possible implications in burn patients. Arch Surg. 1992 Jan;127(1):65–69. doi: 10.1001/archsurg.1992.01420010079011. [DOI] [PubMed] [Google Scholar]
- Zhou D., Munster A., Winchurch R. A. Pathologic concentrations of interleukin 6 inhibit T cell responses via induction of activation of TGF-beta. FASEB J. 1991 Aug;5(11):2582–2585. doi: 10.1096/fasebj.5.11.1868982. [DOI] [PubMed] [Google Scholar]
- de Benedetti F., Massa M., Robbioni P., Ravelli A., Burgio G. R., Martini A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1158–1163. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B., Vitters E., van de Putte L. B. Quantitation of glycosaminoglycan metabolism in anatomically intact articular cartilage of the mouse patella: in vitro and in vivo studies with 35S-sulfate, 3H-glucosamine, and 3H-acetate. Rheumatol Int. 1986;6(6):273–281. doi: 10.1007/BF00541319. [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J., van den Berg W. B. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis. 1994 Sep;53(9):593–600. doi: 10.1136/ard.53.9.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen M. A., Westra J., Limburg P. C., van Riel P. L., van Rijswijk M. H. Clinical significance of interleukin-6 measurement in early rheumatoid arthritis: relation with laboratory and clinical variables and radiological progression in a three year prospective study. Ann Rheum Dis. 1995 Aug;54(8):674–677. doi: 10.1136/ard.54.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen M. A., Westra J., Limburg P. C., van Riel P. L., van Rijswijk M. H. Interleukin-6 in relation to other proinflammatory cytokines, chemotactic activity and neutrophil activation in rheumatoid synovial fluid. Ann Rheum Dis. 1995 Jan;54(1):33–38. doi: 10.1136/ard.54.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lent P. L., van den Berg W. B., Schalkwijk J., van de Putte L. B., van den Bersselaar L. The impact of protein size and charge on its retention in articular cartilage. J Rheumatol. 1987 Aug;14(4):798–805. [PubMed] [Google Scholar]
- van Osch G. J., van der Kraan P. M., van Valburg A. A., van den Berg W. B. The relation between cartilage damage and osteophyte size in a murine model for osteoarthritis in the knee. Rheumatol Int. 1996;16(3):115–119. doi: 10.1007/BF01409983. [DOI] [PubMed] [Google Scholar]
- van Roon J. A., van Roy J. L., Gmelig-Meyling F. H., Lafeber F. P., Bijlsma J. W. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996 May;39(5):829–835. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]
- van de Loo A. A., Arntz O. J., Bakker A. C., van Lent P. L., Jacobs M. J., van den Berg W. B. Role of interleukin 1 in antigen-induced exacerbations of murine arthritis. Am J Pathol. 1995 Jan;146(1):239–249. [PMC free article] [PubMed] [Google Scholar]
- van de Loo F. A., Joosten L. A., van Lent P. L., Arntz O. J., van den Berg W. B. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995 Feb;38(2):164–172. doi: 10.1002/art.1780380204. [DOI] [PubMed] [Google Scholar]
- van der Kraan P. M., Vitters E. L., van Beuningen H. M., van de Putte L. B., van den Berg W. B. Degenerative knee joint lesions in mice after a single intra-articular collagenase injection. A new model of osteoarthritis. J Exp Pathol (Oxford) 1990 Feb;71(1):19–31. [PMC free article] [PubMed] [Google Scholar]
- von Asmuth E. J., Maessen J. G., van der Linden C. J., Buurman W. A. Tumour necrosis factor alpha (TNF-alpha) and interleukin 6 in a zymosan-induced shock model. Scand J Immunol. 1990 Oct;32(4):313–319. doi: 10.1111/j.1365-3083.1990.tb02925.x. [DOI] [PubMed] [Google Scholar]