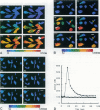
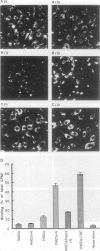
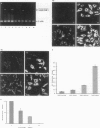
Abstract
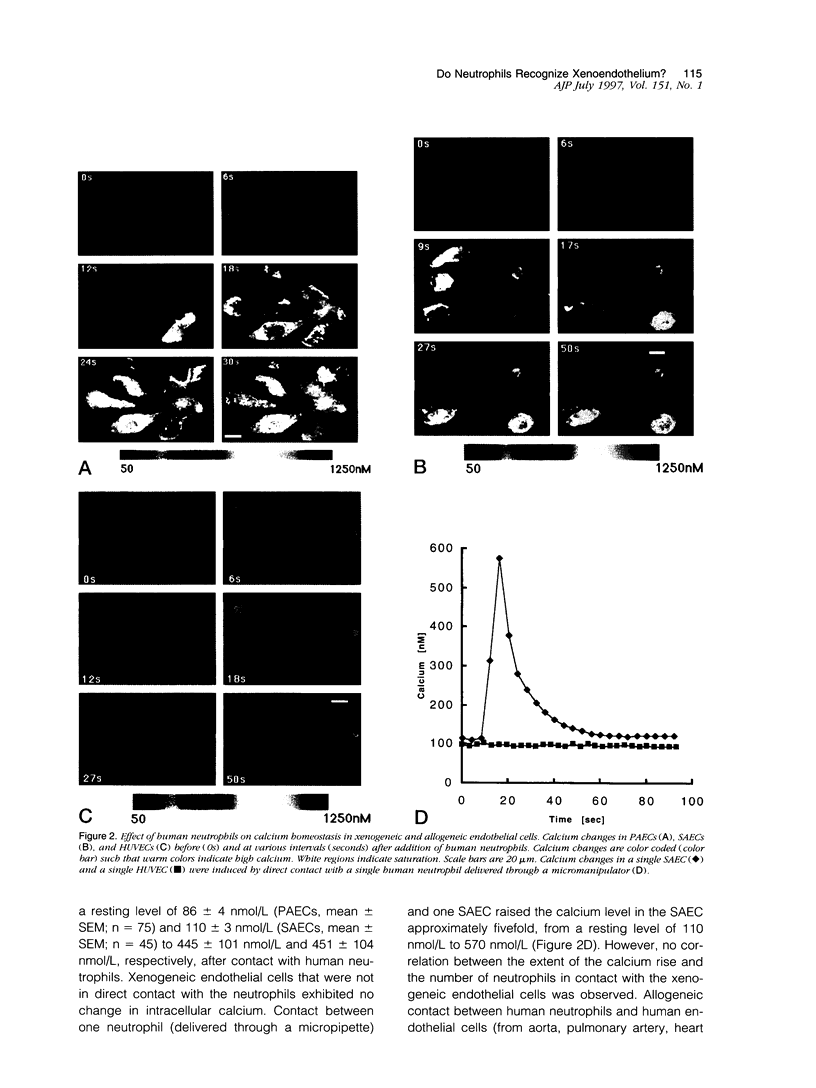
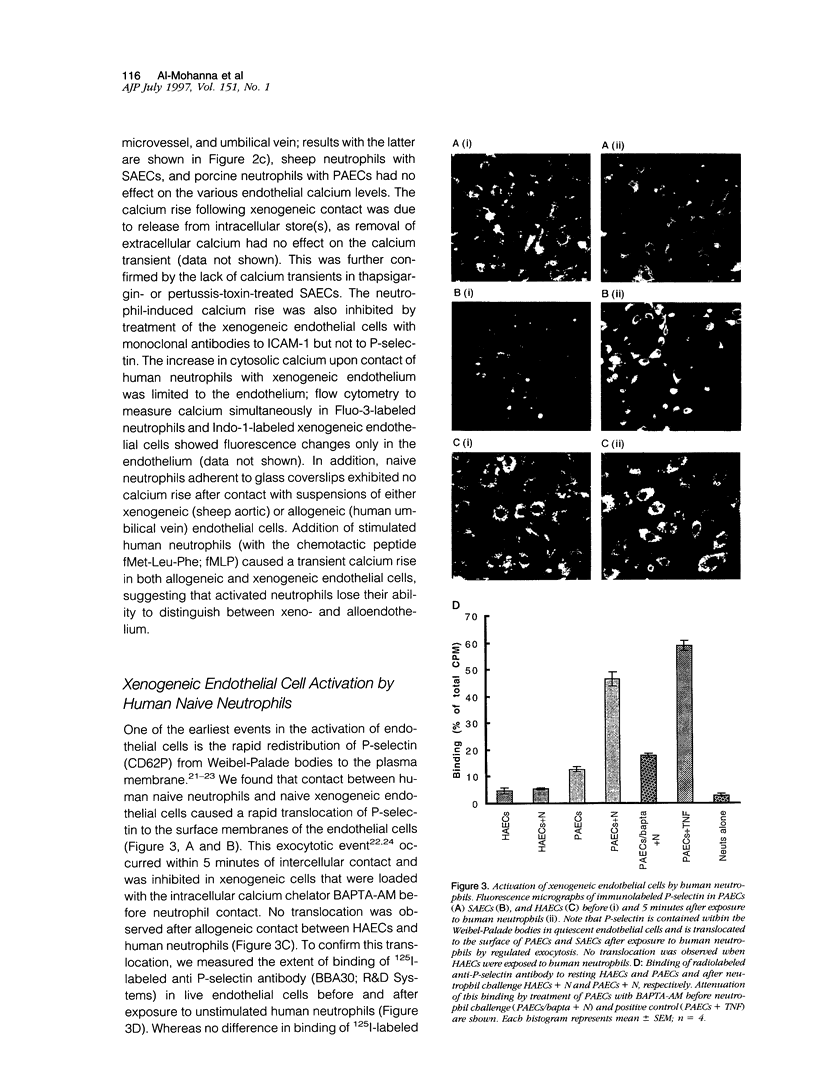
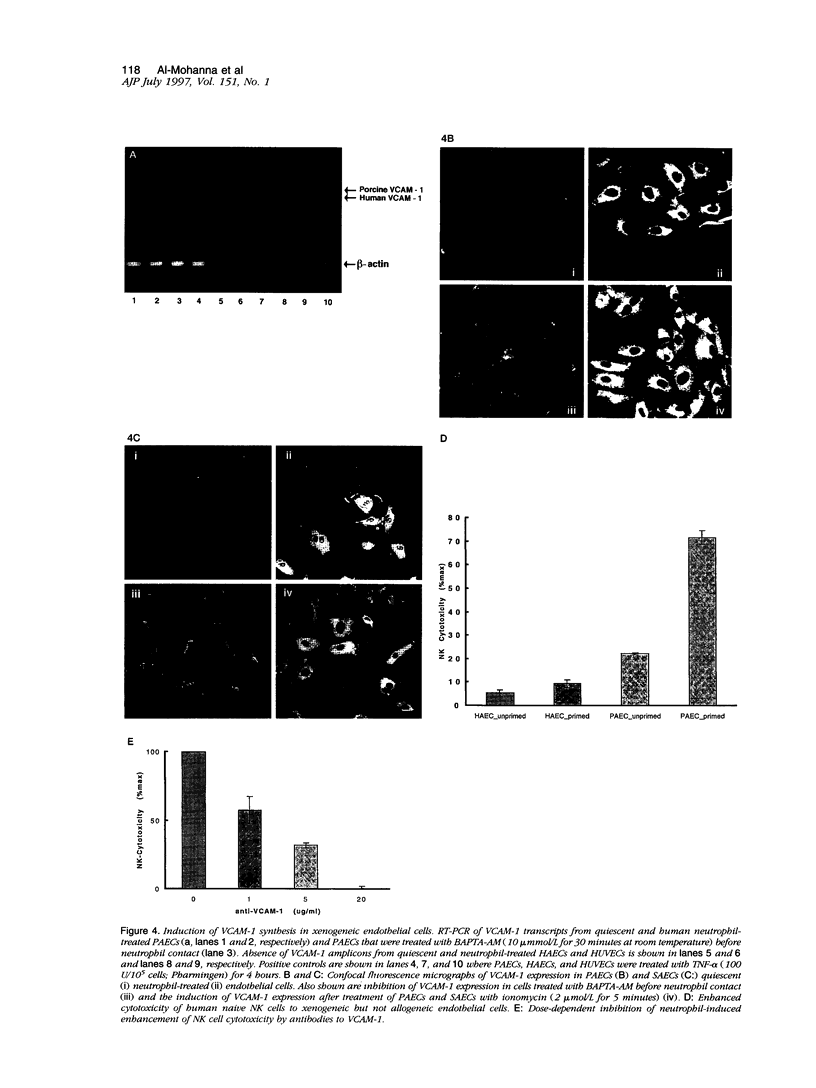
Here we demonstrate that human neutrophils, the predominant circulating leukocytes in intimate contact with endothelial cells lining the vasculature, directly recognize xenogeneic endothelium independently of xenoreactive natural antibody and complement. A rapid and calcium-dependent activation of native (unstimulated) xenogenic endothelial cells by human neutrophils leads to 1) translocation of P-selectin from the Wiebel-Palade bodies to the surface of xenogeneic endothelial cells, 2) increased synthesis and expression of vascular cell adhesion molecule-1 on the xenogeneic endothelial cells, and 3) enhanced killing of the xenogeneic endothelium by natural killer cells. Our data directly implicate naive neutrophils as major early participants in xenograft recognition and endothelial activation independent of xenoreactive natural antibodies and complement.
Full text
PDF


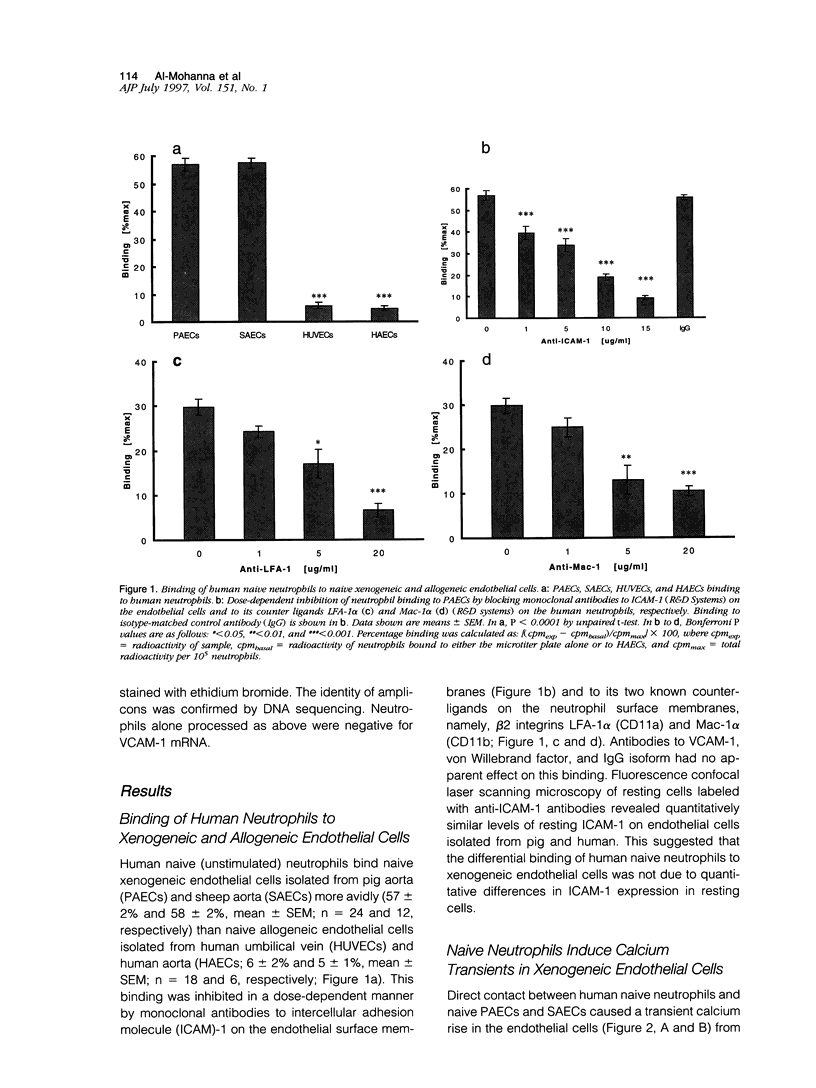



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Mohanna F. A., Hallett M. B. Actin polymerization modifies stimulus-oxidase coupling in rat neutrophils. Biochim Biophys Acta. 1987 Mar 11;927(3):366–371. doi: 10.1016/0167-4889(87)90101-7. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Robson S. C., Ferran C., Millan M., Anrather J., Kopp C., Lesnikoski B., Goodman D. J., Hancock W. W., Wrighton C. Xenotransplantation: endothelial cell activation and beyond. Transplant Proc. 1995 Feb;27(1):77–79. [PubMed] [Google Scholar]
- Bach F. H., Winkler H., Ferran C., Hancock W. W., Robson S. C. Delayed xenograft rejection. Immunol Today. 1996 Aug;17(8):379–384. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- Blakely M. L., Van der Werf W. J., Berndt M. C., Dalmasso A. P., Bach F. H., Hancock W. W. Activation of intragraft endothelial and mononuclear cells during discordant xenograft rejection. Transplantation. 1994 Nov 27;58(10):1059–1066. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. W., McCurry K. R., Martin M. J., McClellan S. M., Platt J. L., Logan J. S. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997 Jan 15;63(1):149–155. doi: 10.1097/00007890-199701150-00027. [DOI] [PubMed] [Google Scholar]
- Collison K. S., Kwaasi A. A., Parhar R. S., al-Sedairy S. T., al-Mohanna F. A. Monomeric human IgE evokes a transient calcium rise in individual human neutrophils. J Leukoc Biol. 1995 Oct;58(4):459–467. doi: 10.1002/jlb.58.4.459. [DOI] [PubMed] [Google Scholar]
- Dalmasso A. P., Vercellotti G. M., Platt J. L., Bach F. H. Inhibition of complement-mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation. 1991 Sep;52(3):530–533. doi: 10.1097/00007890-199109000-00029. [DOI] [PubMed] [Google Scholar]
- Foreman K. E., Vaporciyan A. A., Bonish B. K., Jones M. L., Johnson K. J., Glovsky M. M., Eddy S. M., Ward P. A. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994 Sep;94(3):1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries J. W., Williams A. J., Atkins R. C., Newman W., Lipscomb M. F., Collins T. Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am J Pathol. 1993 Sep;143(3):725–737. [PMC free article] [PubMed] [Google Scholar]
- Geller R. L., Bach F. H., Turman M. A., Casali P., Platt J. L. Evidence that polyreactive antibodies are deposited in rejected discordant xenografts. Transplantation. 1993 Jan;55(1):168–172. doi: 10.1097/00007890-199301000-00031. [DOI] [PubMed] [Google Scholar]
- Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb 22;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Goodman D. J., Von Albertini M., Willson A., Millan M. T., Bach F. H. Direct activation of porcine endothelial cells by human natural killer cells. Transplantation. 1996 Mar 15;61(5):763–771. doi: 10.1097/00007890-199603150-00016. [DOI] [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Huang A. J., Manning J. E., Bandak T. M., Ratau M. C., Hanser K. R., Silverstein S. C. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol. 1993 Mar;120(6):1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T. W., Hakkert B. C., Hoogerwerf M., Leeuwenberg J. F., Roos D. Role of endothelial leukocyte adhesion molecule-1 and platelet-activating factor in neutrophil adherence to IL-1-prestimulated endothelial cells. Endothelial leukocyte adhesion molecule-1-mediated CD18 activation. J Immunol. 1991 Aug 15;147(4):1369–1376. [PubMed] [Google Scholar]
- Lorant D. E., Topham M. K., Whatley R. E., McEver R. P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Inflammatory roles of P-selectin. J Clin Invest. 1993 Aug;92(2):559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks R. M., Todd R. F., 3rd, Ward P. A. Rapid induction of neutrophil-endothelial adhesion by endothelial complement fixation. Nature. 1989 May 25;339(6222):314–317. doi: 10.1038/339314a0. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- Mejía-Laguna J. E., Martínez-Palomo A., López-Soriano F., García-Cornjeo M., Biro C. E. Prolonged survival of kidney xenografts in leucopenic rabbits. Immunology. 1971 Dec;21(6):879–882. [PMC free article] [PubMed] [Google Scholar]
- Minanov O. P., Itescu S., Michler R. E. Recent advances and the potential for clinical use of xenotransplantation. Curr Opin Cardiol. 1996 Mar;11(2):214–220. doi: 10.1097/00001573-199603000-00016. [DOI] [PubMed] [Google Scholar]
- Miyagawa S., Hirose H., Shirakura R., Naka Y., Nakata S., Kawashima Y., Seya T., Matsumoto M., Uenaka A., Kitamura H. The mechanism of discordant xenograft rejection. Transplantation. 1988 Dec;46(6):825–830. doi: 10.1097/00007890-198812000-00007. [DOI] [PubMed] [Google Scholar]
- Parhar R. S., Shi Y., Zou M., Farid N. R., Ernst P., al-Sedairy S. T. Effects of cytokine-mediated modulation of nm23 expression on the invasion and metastatic behavior of B16F10 melanoma cells. Int J Cancer. 1995 Jan 17;60(2):204–210. doi: 10.1002/ijc.2910600213. [DOI] [PubMed] [Google Scholar]
- Parker W., Saadi S., Lin S. S., Holzknecht Z. E., Bustos M., Platt J. L. Transplantation of discordant xenografts: a challenge revisited. Immunol Today. 1996 Aug;17(8):373–378. doi: 10.1016/0167-5699(96)10028-1. [DOI] [PubMed] [Google Scholar]
- Pfau S., Leitenberg D., Rinder H., Smith B. R., Pardi R., Bender J. R. Lymphocyte adhesion-dependent calcium signaling in human endothelial cells. J Cell Biol. 1995 Mar;128(5):969–978. doi: 10.1083/jcb.128.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L. A perspective on xenograft rejection and accommodation. Immunol Rev. 1994 Oct;141:127–149. doi: 10.1111/j.1600-065x.1994.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Lindman B. J., Geller R. L., Noreen H. J., Swanson J. L., Dalmasso A. P., Bach F. H. The role of natural antibodies in the activation of xenogenic endothelial cells. Transplantation. 1991 Dec;52(6):1037–1043. doi: 10.1097/00007890-199112000-00019. [DOI] [PubMed] [Google Scholar]
- Roush W. New ways to avoid organ rejection buoy hopes. Science. 1995 Oct 13;270(5234):234–235. doi: 10.1126/science.270.5234.234. [DOI] [PubMed] [Google Scholar]
- Sciacca F. L., Stürzl M., Bussolino F., Sironi M., Brandstetter H., Zietz C., Zhou D., Matteucci C., Peri G., Sozzani S. Expression of adhesion molecules, platelet-activating factor, and chemokines by Kaposi's sarcoma cells. J Immunol. 1994 Nov 15;153(10):4816–4825. [PubMed] [Google Scholar]
- Vercellotti G. M., Platt J. L., Bach F. H., Dalmasso A. P. Neutrophil adhesion to xenogeneic endothelium via iC3b. J Immunol. 1991 Jan 15;146(2):730–734. [PubMed] [Google Scholar]
- Vischer U. M., Jornot L., Wollheim C. B., Theler J. M. Reactive oxygen intermediates induce regulated secretion of von Willebrand factor from cultured human vascular endothelial cells. Blood. 1995 Jun 1;85(11):3164–3172. [PubMed] [Google Scholar]
- Weber C., Negrescu E., Erl W., Pietsch A., Frankenberger M., Ziegler-Heitbrock H. W., Siess W., Weber P. C. Inhibitors of protein tyrosine kinase suppress TNF-stimulated induction of endothelial cell adhesion molecules. J Immunol. 1995 Jul 1;155(1):445–451. [PubMed] [Google Scholar]
- Young V. K., Kaspar-König W., Tew D. N., Wallwork J., White D. J., Pierson R. N., 3rd White blood cells and platelets are integral to the hyperacute rejection of the pig heart by human blood. Transplant Proc. 1995 Feb;27(1):272–273. [PubMed] [Google Scholar]
- Ziegelstein R. C., Corda S., Pili R., Passaniti A., Lefer D., Zweier J. L., Fraticelli A., Capogrossi M. C. Initial contact and subsequent adhesion of human neutrophils or monocytes to human aortic endothelial cells releases an endothelial intracellular calcium store. Circulation. 1994 Oct;90(4):1899–1907. doi: 10.1161/01.cir.90.4.1899. [DOI] [PubMed] [Google Scholar]