Abstract
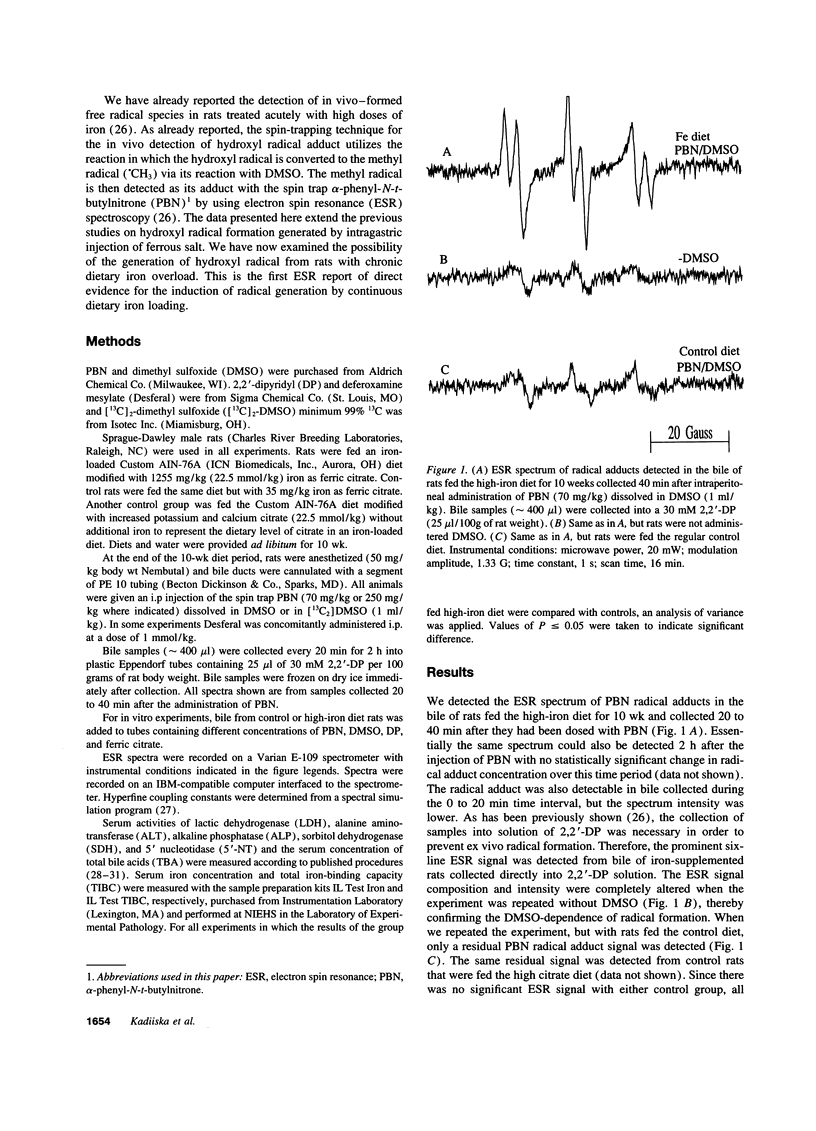
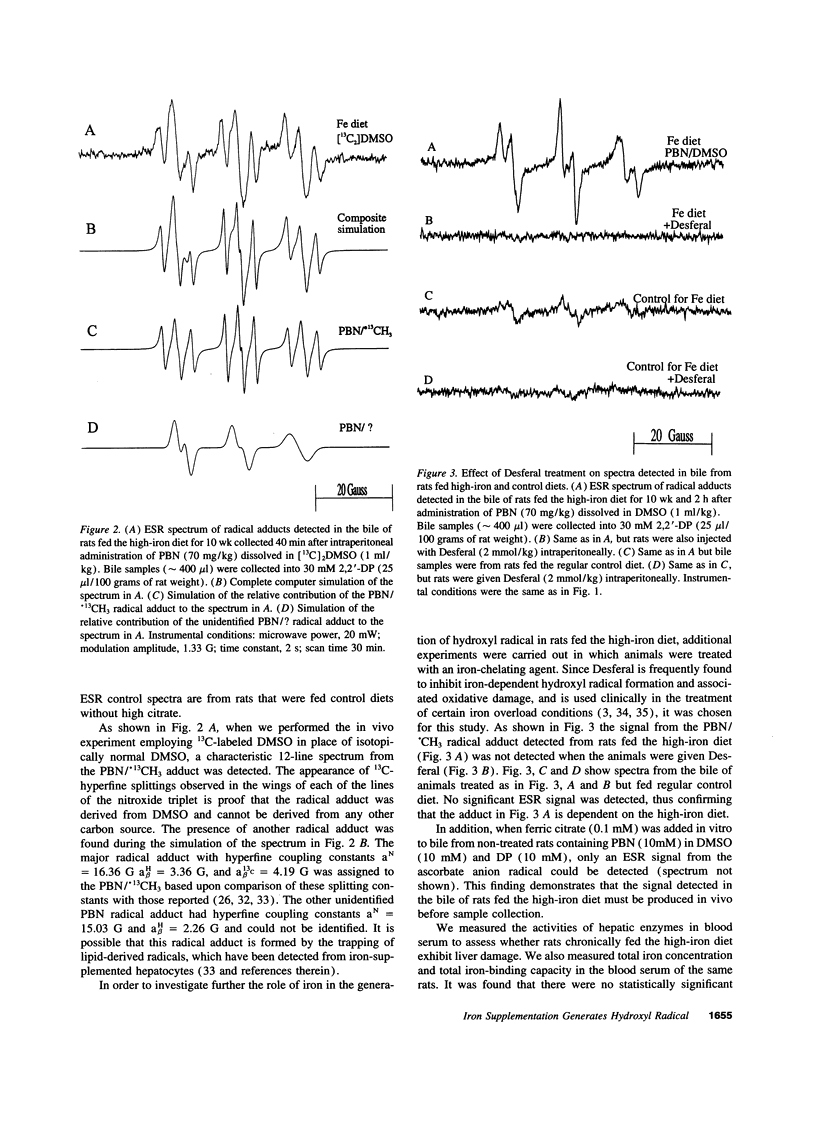
Electron spin resonance (ESR) spectroscopy has been used to investigate hydroxyl radical generation in rats with chronic dietary iron loading. A secondary radical spin-trapping technique was used where hydroxyl radical forms methyl radical upon reaction with DMSO. The methyl radical was then detected by ESR spectroscopy as its adduct with the spin trap alpha-phenyl-N-t-butylnitrone (PBN). This adduct was detected in the bile of rats 10 wk after being fed an iron-loading diet and 40 min after the i.p. injection of the spin trap PBN dissolved in DMSO. Bile samples were collected into a solution of the ferrous stabilizing chelator 2,2'-dipyridyl in order to prevent the generation of radical adducts ex vivo during bile collection. Identification of the ESR spectrum of the major radical adduct as that of PBN/.CH3 provides evidence for the generation of the hydroxyl radical during iron supplementation. Desferal completely inhibited in vivo hydroxyl radical generation stimulated by high dietary iron intake. No radical adducts were detected in rats which were fed the control diet for the same period of time. This is the first evidence of hydroxyl radical generation in chronic iron-loaded rats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Cohen G., Kang J. O. Iron toxicosis. Int Rev Exp Pathol. 1990;31:1–46. doi: 10.1016/b978-0-12-364931-7.50006-9. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- BOTHWELL T. H., ISAACSON C. Siderosis in the bantu. A comparison of incidence in males and females. Br Med J. 1962 Feb 24;1(5277):522–524. doi: 10.1136/bmj.1.5277.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon B. R., Brittenham G. M., Tavill A. S., McLaren C. E., Park C. H., Recknagel R. O. Hepatic lipid peroxidation in vivo in rats with chronic dietary iron overload is dependent on hepatic iron concentration. Trans Assoc Am Physicians. 1983;96:146–154. [PubMed] [Google Scholar]
- Bacon B. R., Britton R. S. Hepatic injury in chronic iron overload. Role of lipid peroxidation. Chem Biol Interact. 1989;70(3-4):183–226. doi: 10.1016/0009-2797(89)90045-8. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Britton R. S. The pathology of hepatic iron overload: a free radical--mediated process? Hepatology. 1990 Jan;11(1):127–137. doi: 10.1002/hep.1840110122. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Tavill A. S., Brittenham G. M., Park C. H., Recknagel R. O. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983 Mar;71(3):429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. S., Gebicki J. M. The effect of pH on yields of hydroxyl radicals produced from superoxide by potential biological iron chelators. Arch Biochem Biophys. 1986 May 1;246(2):581–588. doi: 10.1016/0003-9861(86)90313-9. [DOI] [PubMed] [Google Scholar]
- Bassett M. L., Halliday J. W., Powell L. W. Genetic hemochromatosis. Semin Liver Dis. 1984 Aug;4(3):217–227. doi: 10.1055/s-2008-1041772. [DOI] [PubMed] [Google Scholar]
- Britton R. S., Bacon B. R., Recknagel R. O. Lipid peroxidation and associated hepatic organelle dysfunction in iron overload. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):207–239. doi: 10.1016/0009-3084(87)90066-1. [DOI] [PubMed] [Google Scholar]
- Burkitt M. J., Gilbert B. C. The autoxidation of iron(II) in aqueous systems: the effects of iron chelation by physiological, non-physiological and therapeutic chelators on the generation of reactive oxygen species and the inducement of biomolecular damage. Free Radic Res Commun. 1991;14(2):107–123. doi: 10.3109/10715769109094123. [DOI] [PubMed] [Google Scholar]
- Burkitt M. J., Kadiiska M. B., Hanna P. M., Jordan S. J., Mason R. P. Electron spin resonance spin-trapping investigation into the effects of paraquat and desferrioxamine on hydroxyl radical generation during acute iron poisoning. Mol Pharmacol. 1993 Feb;43(2):257–263. [PubMed] [Google Scholar]
- Burkitt M. J., Mason R. P. Direct evidence for in vivo hydroxyl-radical generation in experimental iron overload: an ESR spin-trapping investigation. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8440–8444. doi: 10.1073/pnas.88.19.8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duling D. R. Simulation of multiple isotropic spin-trap EPR spectra. J Magn Reson B. 1994 Jun;104(2):105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- GOLBERG L., MARTIN L. E., BATCHELOR A. Biochemical changes in the tissues of animals injected with iron. 3. Lipid peroxidation. Biochem J. 1962 May;83:291–298. doi: 10.1042/bj0830291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordeuk V. R., Bacon B. R., Brittenham G. M. Iron overload: causes and consequences. Annu Rev Nutr. 1987;7:485–508. doi: 10.1146/annurev.nu.07.070187.002413. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Hydroxyl radical formation from the auto-reduction of a ferric citrate complex. Free Radic Biol Med. 1991;11(4):401–406. doi: 10.1016/0891-5849(91)90157-x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Superoxide-dependent formation of hydroxyl radicals from ferric-complexes and hydrogen peroxide: an evaluation of fourteen iron chelators. Free Radic Res Commun. 1990;9(2):119–125. doi: 10.3109/10715769009148579. [DOI] [PubMed] [Google Scholar]
- Harris D. C., Aisen P. Facilitation of Fe(II) autoxidation by Fe(3) complexing agents. Biochim Biophys Acta. 1973 Nov 2;329(1):156–158. doi: 10.1016/0304-4165(73)90019-6. [DOI] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- Mak I. T., Weglicki W. B. Characterization of iron-mediated peroxidative injury in isolated hepatic lysosomes. J Clin Invest. 1985 Jan;75(1):58–63. doi: 10.1172/JCI111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashige F., Tanaka N., Maki A., Kamei S., Yamanaka M. Direct spectrophotometry of total bile acids in serum. Clin Chem. 1981 Aug;27(8):1352–1356. [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McLaren G. D., Muir W. A., Kellermeyer R. W. Iron overload disorders: natural history, pathogenesis, diagnosis, and therapy. Crit Rev Clin Lab Sci. 1983;19(3):205–266. doi: 10.3109/10408368309165764. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. An investigation into the mechanism of citrate-Fe2+-dependent lipid peroxidation. Free Radic Biol Med. 1987;3(6):379–387. doi: 10.1016/0891-5849(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. Superoxide-dependent redox cycling of citrate-Fe3+: evidence for a superoxide dismutaselike activity. Arch Biochem Biophys. 1987 Feb 15;253(1):257–267. doi: 10.1016/0003-9861(87)90659-x. [DOI] [PubMed] [Google Scholar]
- Minotti G., Di Gennaro M., D'Ugo D., Granone P. Possible sources of iron for lipid peroxidation. Free Radic Res Commun. 1991;12-13 Pt 1:99–106. doi: 10.3109/10715769109145773. [DOI] [PubMed] [Google Scholar]
- Morel I., Sergent O., Cogrel P., Lescoat G., Pasdeloup N., Brissot P., Cillard P., Cillard J. EPR study of antioxidant activity of the iron chelators pyoverdin and hydroxypyrid-4-one in iron-loaded hepatocyte culture: comparison with that of desferrioxamine. Free Radic Biol Med. 1995 Feb;18(2):303–310. doi: 10.1016/0891-5849(94)e0144-8. [DOI] [PubMed] [Google Scholar]
- Mulligan M., Althaus B., Linder M. C. Non-ferritin, non-heme iron pools in rat tissues. Int J Biochem. 1986;18(9):791–798. doi: 10.1016/0020-711x(86)90055-8. [DOI] [PubMed] [Google Scholar]
- Niederau C., Fischer R., Sonnenberg A., Stremmel W., Trampisch H. J., Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985 Nov 14;313(20):1256–1262. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Ward R. J., Baum H., Peters T. J. The role of iron in ferritin- and haemosiderin-mediated lipid peroxidation in liposomes. Biochem J. 1985 Jul 1;229(1):135–139. doi: 10.1042/bj2290135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Steinberg D., Witztum J. L. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- Powell L. W., Bassett M. L., Halliday J. W. Hemochromatosis: 1980 update. Gastroenterology. 1980 Feb;78(2):374–381. [PubMed] [Google Scholar]
- Puntarulo S., Cederbaum A. I. Comparison of the ability of ferric complexes to catalyze microsomal chemiluminescence, lipid peroxidation, and hydroxyl radical generation. Arch Biochem Biophys. 1988 Aug 1;264(2):482–491. doi: 10.1016/0003-9861(88)90313-x. [DOI] [PubMed] [Google Scholar]
- Reif D. W. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med. 1992;12(5):417–427. doi: 10.1016/0891-5849(92)90091-t. [DOI] [PubMed] [Google Scholar]
- Risdon R. A., Barry M., Flynn D. M. Transfusional iron overload: the relationship between tissue iron concentration and hepatic fibrosis in thalassaemia. J Pathol. 1975 Jun;116(2):83–95. doi: 10.1002/path.1711160204. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Nyyssönen K., Korpela H., Tuomilehto J., Seppänen R., Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992 Sep;86(3):803–811. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- Szweda L. I., Stadtman E. R. Oxidative modification of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides by an iron(II)-citrate complex. Arch Biochem Biophys. 1993 Mar;301(2):391–395. doi: 10.1006/abbi.1993.1161. [DOI] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Vile G. F., Winterbourn C. C., Sutton H. C. Radical-driven Fenton reactions: studies with paraquat, adriamycin, and anthraquinone 6-sulfonate and citrate, ATP, ADP, and pyrophosphate iron chelates. Arch Biochem Biophys. 1987 Dec;259(2):616–626. doi: 10.1016/0003-9861(87)90528-5. [DOI] [PubMed] [Google Scholar]
- Voogd A., Sluiter W., van Eijk H. G., Koster J. F. Low molecular weight iron and the oxygen paradox in isolated rat hearts. J Clin Invest. 1992 Nov;90(5):2050–2055. doi: 10.1172/JCI116086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub L. R., Goral A., Grasso J., Franzblau C., Sullivan A., Sullivan S. Pathogenesis of hepatic fibrosis in experimental iron overload. Br J Haematol. 1985 Feb;59(2):321–331. doi: 10.1111/j.1365-2141.1985.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki I., Piette L. H. ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J Biol Chem. 1990 Aug 15;265(23):13589–13594. [PubMed] [Google Scholar]


