Abstract
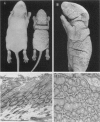
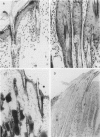
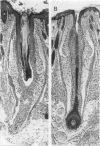
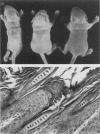
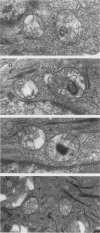
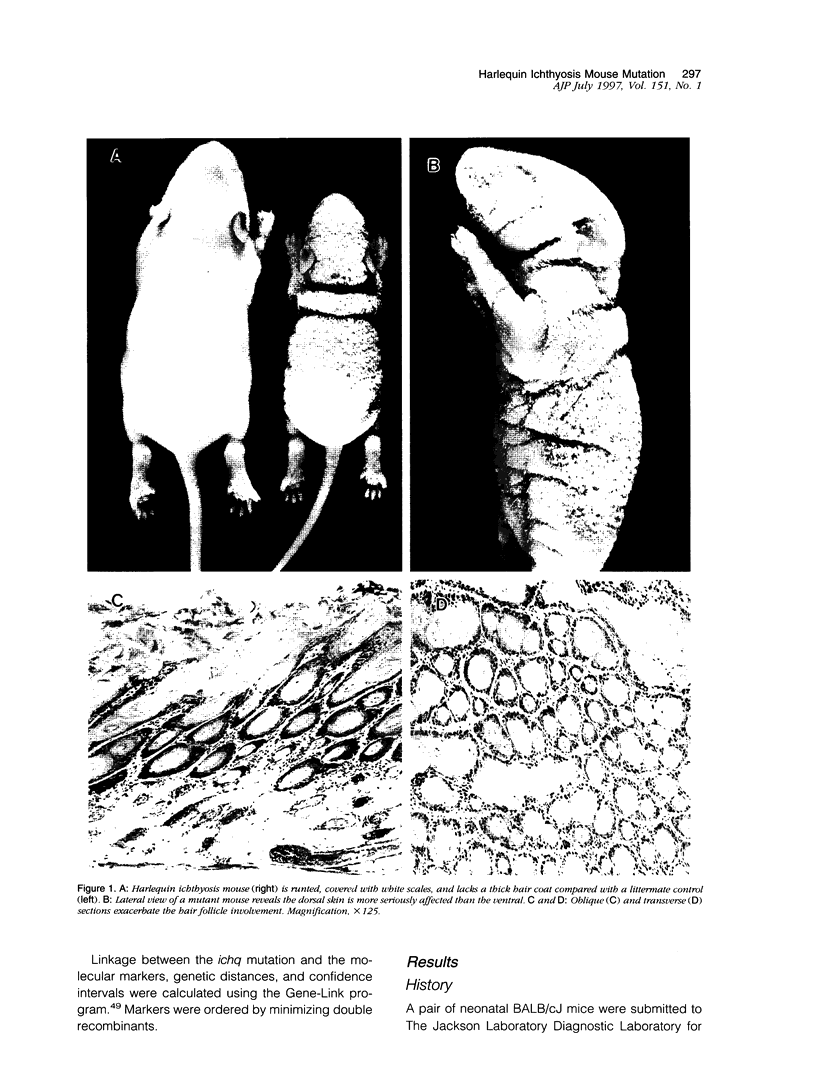
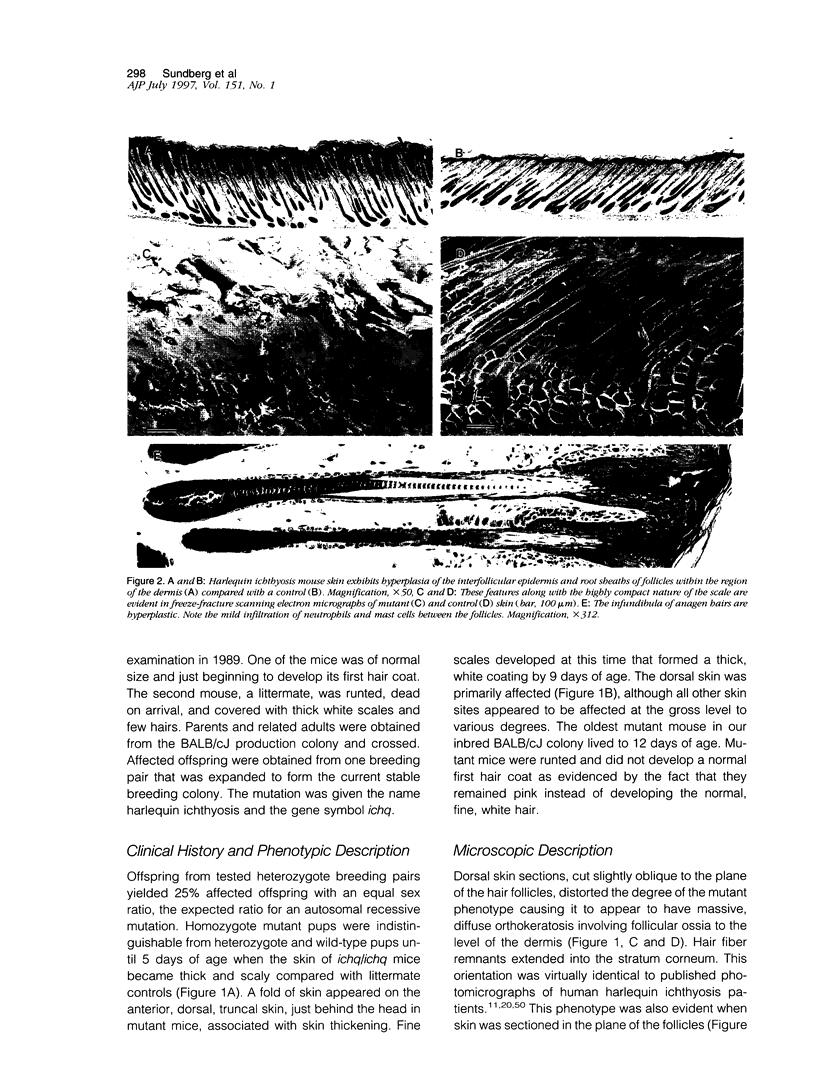
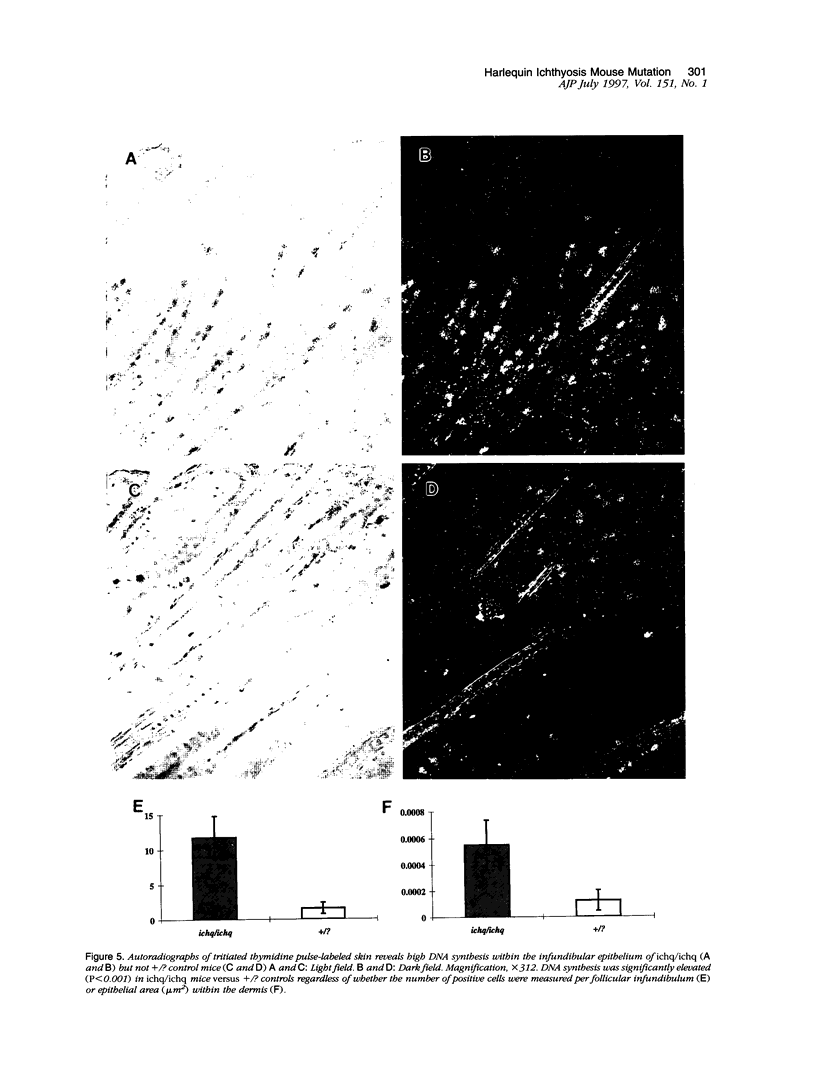
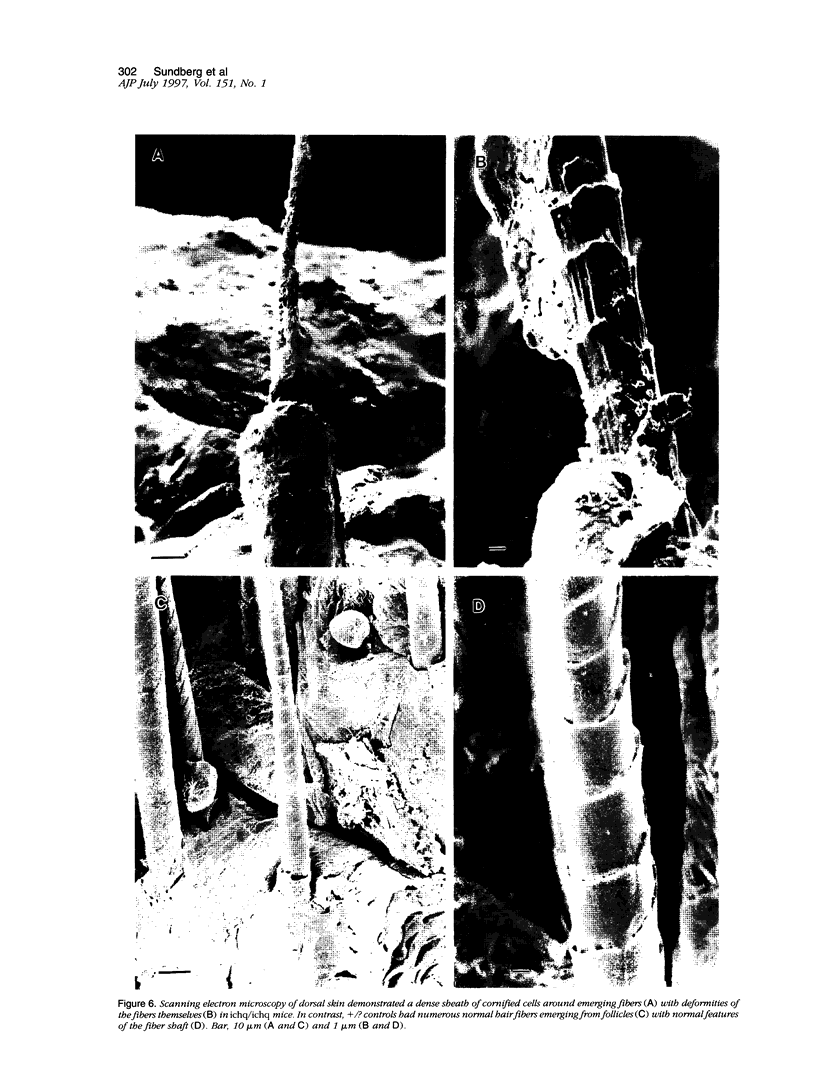
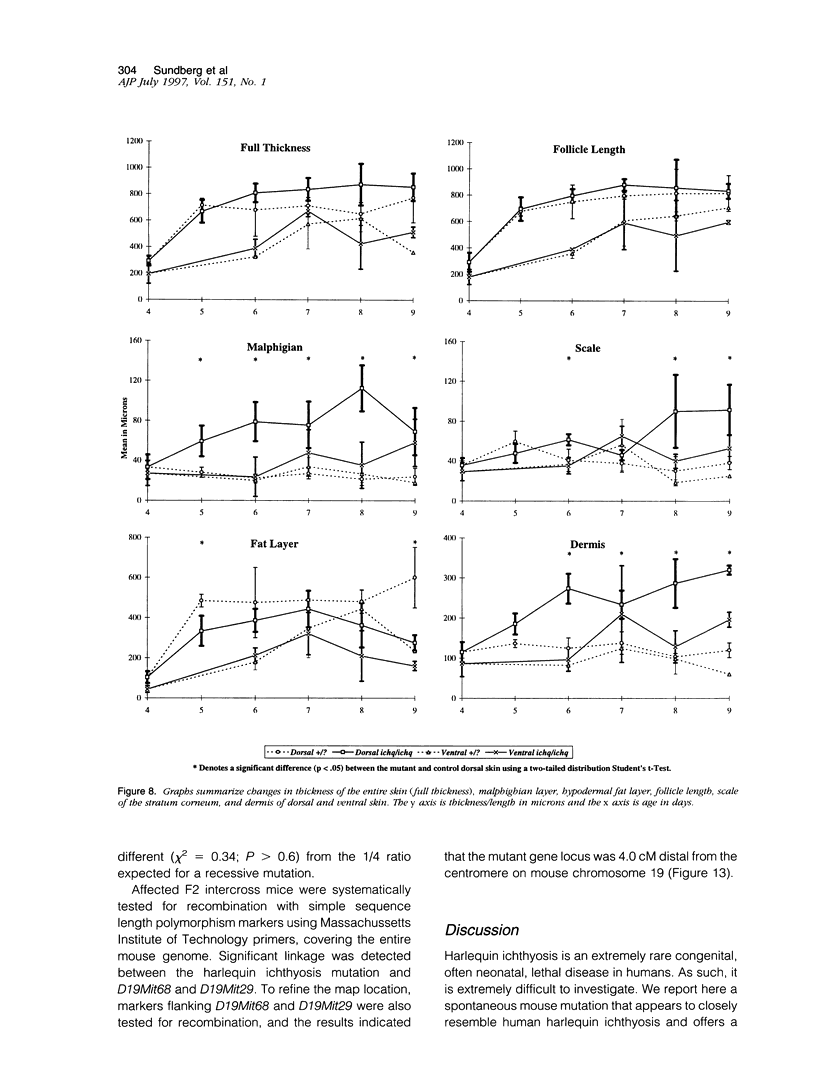
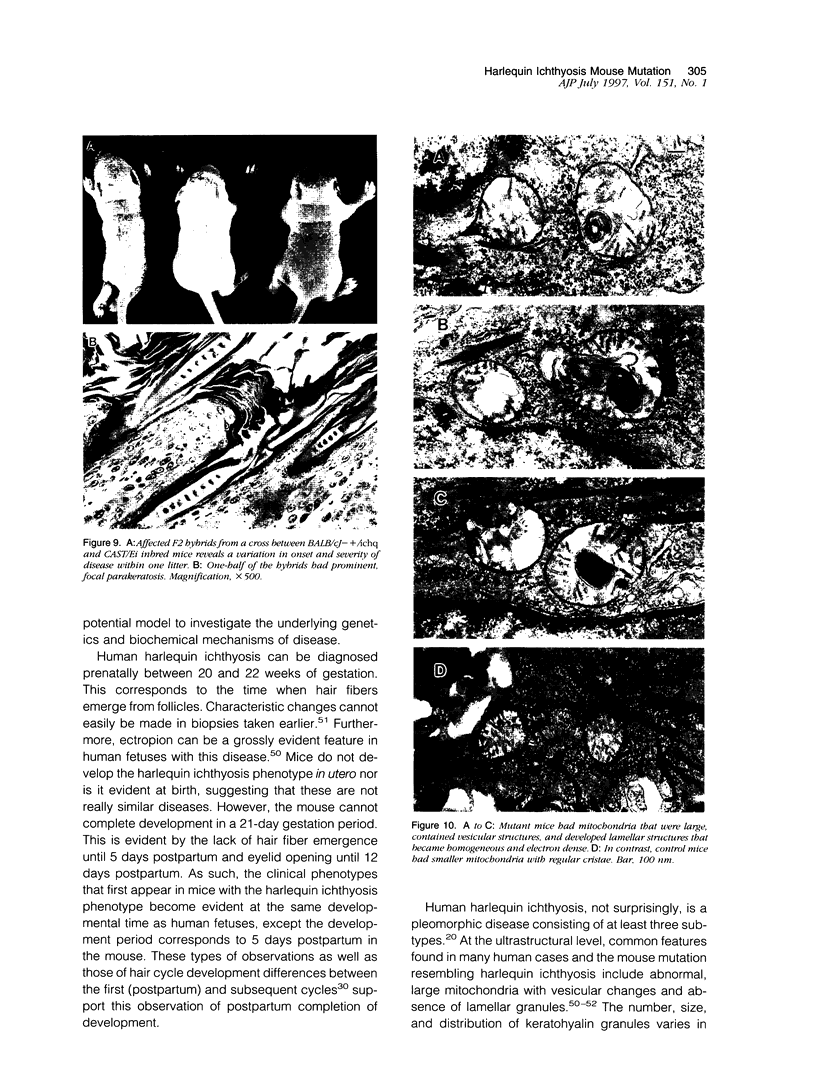
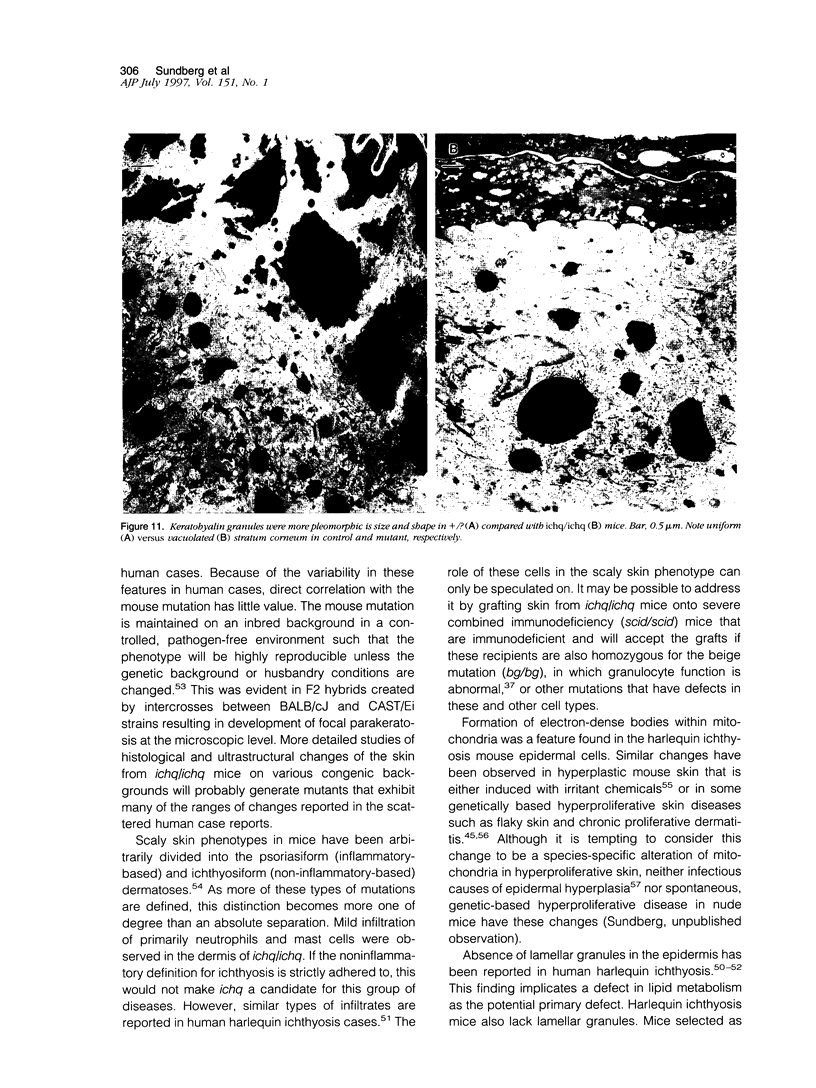
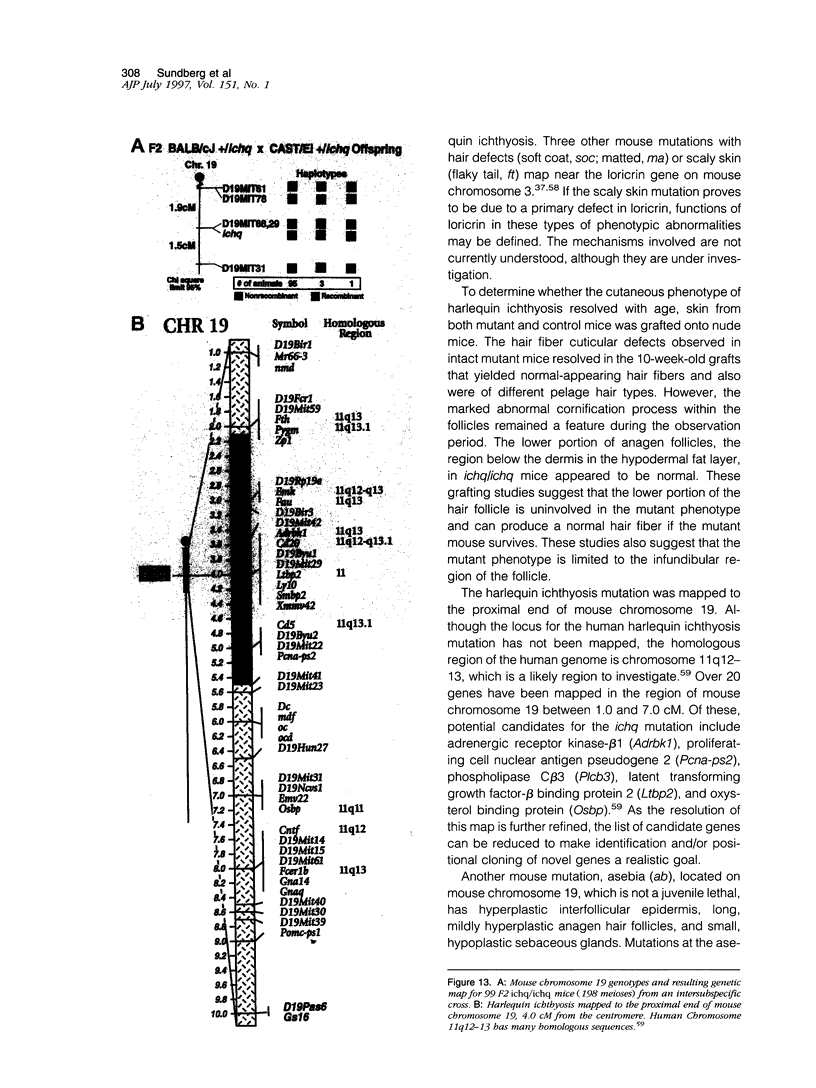
The harlequin ichthyosis (ichq) mouse mutation arose spontaneously in 1989 in a colony of BALB/cJ mice at The Jackson Laboratory. Affected mice developed thick skin due to formation of compact, orthokeratotic scales that fractured over articular surfaces, secondary to bending. Harlequin ichthyosis mice on the inbred BALB/cJ background died between 9 and 12 days of age. Onset of the clinical phenotype corresponded with emergence of hair fibers from follicles at 5 days of age. There was marked proliferation of the root sheaths of anagen hair follicles, limited to the region within the dermis. Sebaceous glands were present but small compared with those of littermate controls. Emerging hair fibers were surrounded by a thick, compact sheath of cornified cells. Mutant skin contained large mitochondria with lamellar-shaped, electron-dense structures at the ultrastructural level. Keratohyalin granules were smaller and less pleomorphic than those in control mice. Lamellar bodies were not evident in either mutant or littermate control mice. Using a panel of antibodies to evaluate changes in keratinocyte differentiation, mouse-specific keratin 6 was overexpressed in the suprabasilar, hyperplastic epidermis. Loricrin expression, within the cytoplasm of cells in the stratum granulosum, decreased rapidly postmortem, unlike that in normal mice where it was stable for over 24 hours postmortem. Filaggrin expression, within granules of cells in the stratum granulosum, was prominent, corresponding to hypergranulosis evident by light microscopy in mutant mouse skin. Skin grafts from harlequin ichthyosis mice grafted onto immunodeficient nude mice maintained the phenotype for the 10-week observation period. The mutant gene locus mapped to the proximal end of mouse chromosome 19 and is inherited as a fully penetrant autosomal recessive gene. The harlequin ichthyosis mouse mutation is very similar to human type 2 harlequin ichthyosis for which it may be a good model.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson A., Sperling R., Moshirpur J. Harlequin fetus in twins. Mt Sinai J Med. 1984 Jun;51(3):290–291. [PubMed] [Google Scholar]
- BARKER L. P., SACHS W. Bullous congenital ichthyosiform erythroderma. AMA Arch Derm Syphilol. 1953 May;67(5):443–455. doi: 10.1001/archderm.1953.01540050007002. [DOI] [PubMed] [Google Scholar]
- BRICENO-MAAZ T. Two cases of congenital ichthyosis. Arch Dermatol. 1963 Feb;87:230–233. doi: 10.1001/archderm.1963.01590140092015. [DOI] [PubMed] [Google Scholar]
- Baden H. P., Kubilus J., Rosenbaum K., Fletcher A. Keratinization in the harlequin fetus. Arch Dermatol. 1982 Jan;118(1):14–18. [PubMed] [Google Scholar]
- Baker J. R., Ward W. R. Ichthyosis in domestic animals: a review of the literature and a case report. Br Vet J. 1985 Jan-Feb;141(1):1–8. doi: 10.1016/0007-1935(85)90120-4. [DOI] [PubMed] [Google Scholar]
- Blanchet-Bardon C., Dumez Y., Labbe F., Bernheim A., Brocheriou C. Diagnostic prénatal par microscopie électronique d'un foetus Arlequin. Ann Pathol. 1983 Dec;3(4):321–325. [PubMed] [Google Scholar]
- Clifford C. B., Walton B. J., Reed T. H., Coyle M. B., White W. J., Amyx H. L. Hyperkeratosis in athymic nude mice caused by a coryneform bacterium: microbiology, transmission, clinical signs, and pathology. Lab Anim Sci. 1995 Apr;45(2):131–139. [PubMed] [Google Scholar]
- Craig J. M., Goldsmith L. A., Baden H. P. An abnormality of keratin in the harlequin fetus. Pediatrics. 1970 Sep;46(3):437–440. [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Fleckman P., Kimball J. R., Brumbaugh S., Sybert V. P. Heterogeneity in harlequin ichthyosis, an inborn error of epidermal keratinization: variable morphology and structural protein expression and a defect in lamellar granules. J Invest Dermatol. 1990 Jan;94(1):6–18. doi: 10.1111/1523-1747.ep12873301. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan R. W., Rosser E. J., Jr Newly recognized and emerging genodermatoses in domestic animals. Curr Probl Dermatol. 1987;17:216–235. doi: 10.1159/000413485. [DOI] [PubMed] [Google Scholar]
- Elias S., Mazur M., Sabbagha R., Esterly N. B., Simpson J. L. Prenatal diagnosis of harlequin ichthyosis. Clin Genet. 1980 Apr;17(4):275–280. doi: 10.1111/j.1399-0004.1980.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Esterly N. B. The ichthyosiform dermatoses. Pediatrics. 1968 Dec;42(6):990–1004. [PubMed] [Google Scholar]
- FREI J. V., SHELDON H. Corpus intra cristam: a dense body within mitochondria of cells in hyperplastic mouse epidermis. J Biophys Biochem Cytol. 1961 Dec;11:724–729. doi: 10.1083/jcb.11.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost P., Van Scott E. J. Ichthyosiform dermatoses. Classification based on anatomic and biometric observations. Arch Dermatol. 1966 Aug;94(2):113–126. doi: 10.1001/archderm.94.2.113. [DOI] [PubMed] [Google Scholar]
- Gijbels M. J., HogenEsch H., Blauw B., Roholl P., Zurcher C. Ultrastructure of epidermis of mice with chronic proliferative dermatitis. Ultrastruct Pathol. 1995 Mar-Apr;19(2):107–111. doi: 10.3109/01913129509014610. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., De Dobbeleer G., Kanzaki T. Electron microscopic studies of harlequin fetuses. Pediatr Dermatol. 1993 Sep;10(3):214–223. doi: 10.1111/j.1525-1470.1993.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Khan S. Harlequin fetus with abnormal lamellar granules and giant mitochondria. J Cutan Pathol. 1992 Jun;19(3):247–252. doi: 10.1111/j.1600-0560.1992.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Headington J. T. Transverse microscopic anatomy of the human scalp. A basis for a morphometric approach to disorders of the hair follicle. Arch Dermatol. 1984 Apr;120(4):449–456. [PubMed] [Google Scholar]
- KESSEL I., FRIEDLANDER F. C. Harlequin foetus. Arch Dis Child. 1956 Feb;31(155):53–55. doi: 10.1136/adc.31.155.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor F., Peiris S. Harlequin fetus successfully treated with etretinate. Br J Dermatol. 1985 May;112(5):585–590. doi: 10.1111/j.1365-2133.1985.tb15268.x. [DOI] [PubMed] [Google Scholar]
- Love J. M., Knight A. M., McAleer M. A., Todd J. A. Towards construction of a high resolution map of the mouse genome using PCR-analysed microsatellites. Nucleic Acids Res. 1990 Jul 25;18(14):4123–4130. doi: 10.1093/nar/18.14.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalko M., Lindfors K. K., Grix A. W., Brant W. E., McGahan J. P. Prenatal sonographic diagnosis of harlequin ichthyosis. AJR Am J Roentgenol. 1989 Oct;153(4):827–828. doi: 10.2214/ajr.153.4.827. [DOI] [PubMed] [Google Scholar]
- Milner M. E., O'Guin W. M., Holbrook K. A., Dale B. A. Abnormal lamellar granules in harlequin ichthyosis. J Invest Dermatol. 1992 Dec;99(6):824–829. doi: 10.1111/1523-1747.ep12614791. [DOI] [PubMed] [Google Scholar]
- Montagutelli X. GENE-LINK: a program in PASCAL for backcross genetic analysis. J Hered. 1990 Nov-Dec;81(6):490–491. doi: 10.1093/oxfordjournals.jhered.a111033. [DOI] [PubMed] [Google Scholar]
- Morita K., Hogan M. E., Nanney L. B., King L. E., Jr, Manabe M., Sun T. T., Sundberg J. P. Cutaneous ultrastructural features of the flaky skin (fsn) mouse mutation. J Dermatol. 1995 Jun;22(6):385–395. doi: 10.1111/j.1346-8138.1995.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Roberts L. J. Long-term survival of a harlequin fetus. J Am Acad Dermatol. 1989 Aug;21(2 Pt 2):335–339. doi: 10.1016/s0190-9622(89)80029-5. [DOI] [PubMed] [Google Scholar]
- Rothnagel J. A., Longley M. A., Bundman D. S., Naylor S. L., Lalley P. A., Jenkins N. A., Gilbert D. J., Copeland N. G., Roop D. R. Characterization of the mouse loricrin gene: linkage with profilaggrin and the flaky tail and soft coat mutant loci on chromosome 3. Genomics. 1994 Sep 15;23(2):450–456. doi: 10.1006/geno.1994.1522. [DOI] [PubMed] [Google Scholar]
- Schnyder U. W. Die hereditären Ichthyosen. Schweiz Rundsch Med Prax. 1986 Feb 18;75(8):185–191. [PubMed] [Google Scholar]
- Shwayder T., Ott F. All about ichthyosis. Pediatr Clin North Am. 1991 Aug;38(4):835–857. doi: 10.1016/s0031-3955(16)38156-1. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Hawes N. L., Kuhlmann S. D., Heckenlively J. R., Chang B., Roderick T. H., Sundberg J. P. Corn1: a mouse model for corneal surface disease and neovascularization. Invest Ophthalmol Vis Sci. 1996 Feb;37(2):397–404. [PubMed] [Google Scholar]
- Sundberg J. P., Beamer W. G., Shultz L. D., Dunstan R. W. Inherited mouse mutations as models of human adnexal, cornification, and papulosquamous dermatoses. J Invest Dermatol. 1990 Nov;95(5 Suppl):62S–63S. doi: 10.1111/1523-1747.ep12505816. [DOI] [PubMed] [Google Scholar]
- Sundberg J. P., Dunstan R. W., Roop D. R., Beamer W. G. Full-thickness skin grafts from flaky skin mice to nude mice: maintenance of the psoriasiform phenotype. J Invest Dermatol. 1994 May;102(5):781–788. doi: 10.1111/1523-1747.ep12377741. [DOI] [PubMed] [Google Scholar]
- Sundberg J. P., Erickson A. A., Roop D. R., Binder R. L. Ornithine decarboxylase expression in cutaneous papillomas in SENCAR mice is associated with altered expression of keratins 1 and 10. Cancer Res. 1994 Mar 1;54(5):1344–1351. [PubMed] [Google Scholar]
- Sundberg J. P., King L. E., Jr Mouse mutations as animal models and biomedical tools for dermatological research. J Invest Dermatol. 1996 Feb;106(2):368–376. doi: 10.1111/1523-1747.ep12343152. [DOI] [PubMed] [Google Scholar]
- Sundberg J. P., Schultz L. D. Inherited mouse mutations: models for the study of alopecia. J Invest Dermatol. 1991 May;96(5):95S–96S. doi: 10.1111/1523-1747.ep12472236. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. C., Burnett I. H., Wertz P. W., Madison K. C., Squier C. A. Osmium tetroxide and ruthenium tetroxide are complementary reagents for the preparation of epidermal samples for transmission electron microscopy. J Invest Dermatol. 1995 Mar;104(3):417–420. doi: 10.1111/1523-1747.ep12665909. [DOI] [PubMed] [Google Scholar]
- Unamuno P., Pierola J. M., Fernandez E., Roman C., Velasco J. A. Harlequin foetus in four siblings. Br J Dermatol. 1987 Apr;116(4):569–572. doi: 10.1111/j.1365-2133.1987.tb05880.x. [DOI] [PubMed] [Google Scholar]
- Wells R. S., Kerr C. B. Genetic classification of ichthyosis. Arch Dermatol. 1965 Jul;92(1):1–6. [PubMed] [Google Scholar]
- Whiting D. A. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol. 1993 May;28(5 Pt 1):755–763. doi: 10.1016/0190-9622(93)70106-4. [DOI] [PubMed] [Google Scholar]