Abstract
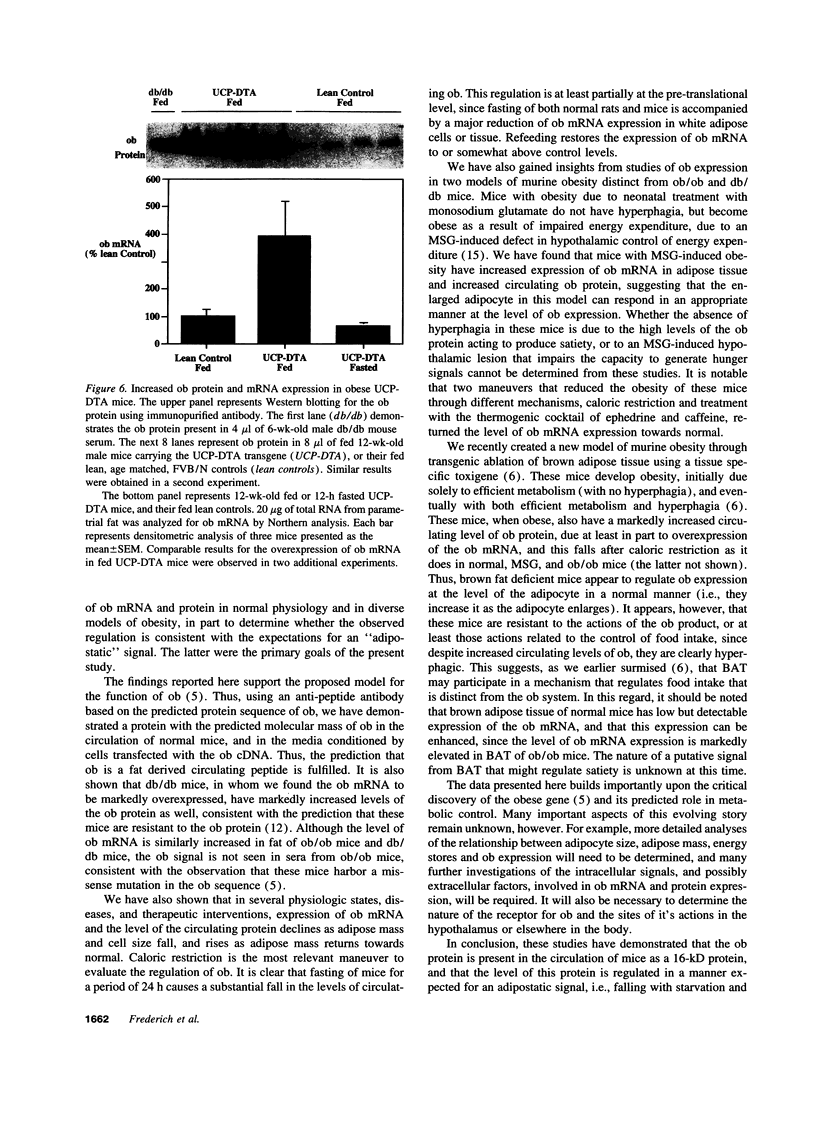
The mutant gene responsible for obesity in the ob/ob mouse was recently identified by positional cloning (Zhang Y., R. Proenca, M. Maffel, M. Barone, L. Leopold, and J.M. Friedman. 1994. Nature (Lond.) 372:425). The encoded protein and to represent and "adipostat" signal reflecting the state of energy stores. We confirm that the adipocyte is the source of ob mRNA and that the predicted 16-kD ob protein is present in rodent serum as detected by Western blot. To evaluate the hypothesis that it might represent an adipostat, we assessed serum levels of ob protein and expression of ob mRNA in adipose cells and tissue of rodents in response to a variety of perturbations which effect body fat mass. Both ob protein and ob mRNA expression are markedly increased in obesity. The levels of ob protein are approximately 5-10-fold elevated in serum of db/db mice, in mice with hypothalamic lesions caused by neonatal administration of monosodium glutamate (MSG), and in mice with toxigene induced brown fat ablation, (UCP-DTA). Very parallel changes are observed in adipocyte ob mRNA expression in these models and in ob/ob mice. As predicted however, no serum ob protein could be detected in the ob/ob mice. By contrast to obesity, starvation of normal rats and mice for 1-3 d markedly suppresses ob mRNA abundance, and this is reversed with refeeding. Similarly, ob protein concentration in normal mice falls to undetectable levels with starvation. In the ob/ob, UCP-DTA and MSG models, overexpression of ob mRNA is reversed by caloric restriction. These data support the hypothesis that expression of ob mRNA and protein are regulated as a function of energy stores, and that ob serves as a circulating feedback signal to sites involved in regulation of energy homeostasis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleary M. P., Brasel J. A., Greenwood M. R. Developmental changes in thymidine kinase, DNA, and fat cellularity in Zucker rats. Am J Physiol. 1979 May;236(5):E508–E513. doi: 10.1152/ajpendo.1979.236.5.E508. [DOI] [PubMed] [Google Scholar]
- Coleman D. L. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973 Aug;9(4):294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Djazayery A., Miller D. S., Stock M. J. Energy balances in obese mice. Nutr Metab. 1979;23(5):357–367. doi: 10.1159/000176281. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Cook K. S., Usher P., Spiegelman B. M. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987 Jul 24;237(4813):405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- Flier J. S. The adipocyte: storage depot or node on the energy information superhighway? Cell. 1995 Jan 13;80(1):15–18. doi: 10.1016/0092-8674(95)90445-x. [DOI] [PubMed] [Google Scholar]
- Hervey G. R. Regulation of energy balance. Nature. 1969 May 17;222(5194):629–631. doi: 10.1038/222629a0. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Mechanism for markedly hyperresponsive insulin-stimulated glucose transport activity in adipose cells from insulin-treated streptozotocin diabetic rats. Evidence for increased glucose transporter intrinsic activity. J Biol Chem. 1987 Apr 15;262(11):5118–5124. [PubMed] [Google Scholar]
- Kahn B. B., Simpson I. A., Cushman S. W. Divergent mechanisms for the insulin resistant and hyperresponsive glucose transport in adipose cells from fasted and refed rats. Alterations in both glucose transporter number and intrinsic activity. J Clin Invest. 1988 Aug;82(2):691–699. doi: 10.1172/JCI113649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell B. B., Napolitano A., Usher P., Dulloo A. G., Rosen B. S., Spiegelman B. M., Flier J. S. Reduced adipsin expression in murine obesity: effect of age and treatment with the sympathomimetic-thermogenic drug mixture ephedrine and caffeine. Endocrinology. 1990 Mar;126(3):1514–1520. doi: 10.1210/endo-126-3-1514. [DOI] [PubMed] [Google Scholar]
- Lowell B. B., S-Susulic V., Hamann A., Lawitts J. A., Himms-Hagen J., Boyer B. B., Kozak L. P., Flier J. S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993 Dec 23;366(6457):740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Tokuyama K., Himms-Hagen J. Brown adipose tissue thermogenesis, torpor, and obesity of glutamate-treated mice. Am J Physiol. 1986 Oct;251(4 Pt 1):E407–E415. doi: 10.1152/ajpendo.1986.251.4.E407. [DOI] [PubMed] [Google Scholar]
- Weigle D. S. Appetite and the regulation of body composition. FASEB J. 1994 Mar 1;8(3):302–310. doi: 10.1096/fasebj.8.3.8143936. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]