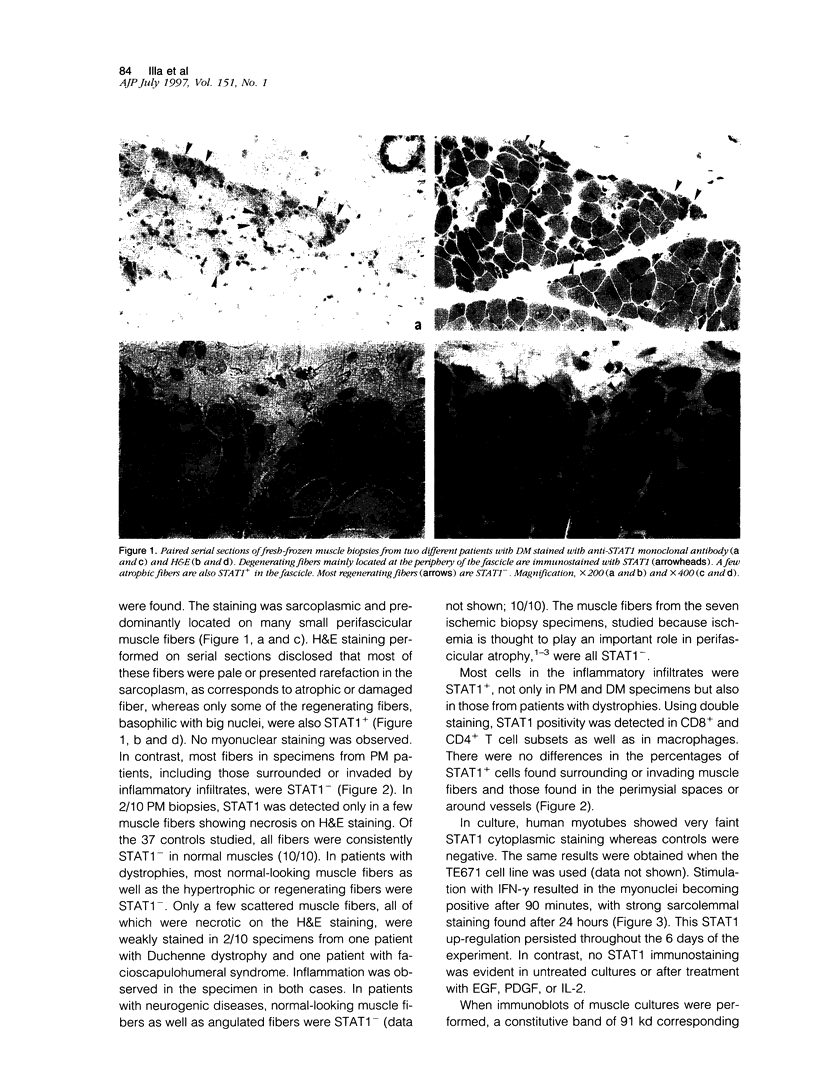
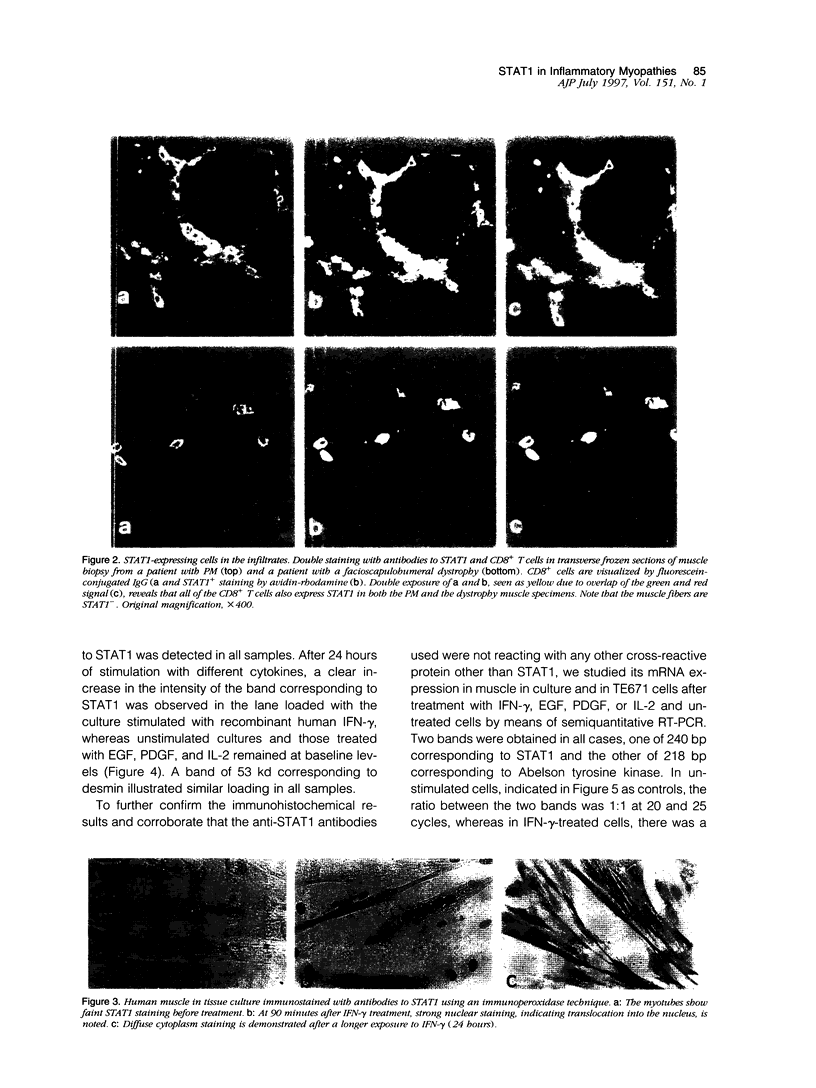
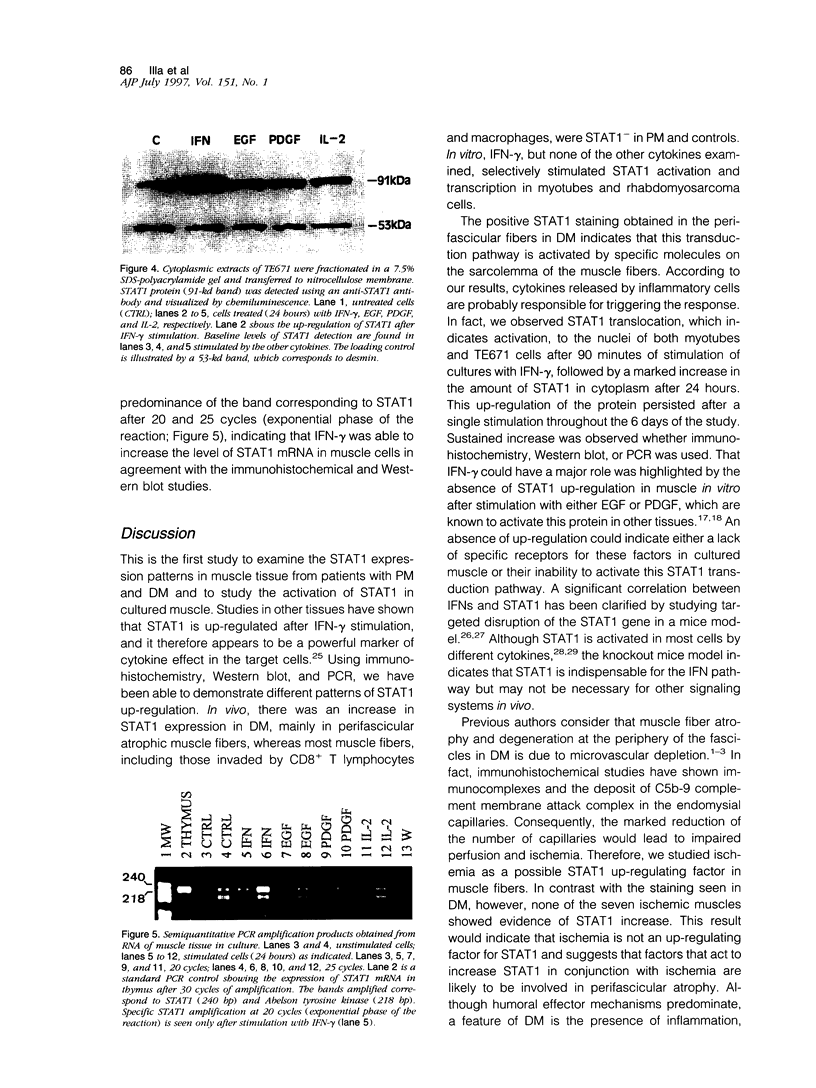
Abstract
Polymyositis (PM) and dermatomyositis (DM) are two major and distinct inflammatory myopathies. Cytokines, implicated in the immune process, have been recognized in the muscle tissue from PM and DM patients, but their functional in situ role has not been identified. We analyzed the expression of the signal transducer and activator of transcription 1 (STAT1), a molecule whose up-regulation indicates the interaction of cytokines, or growth factors, with their target receptors in muscle fibers and inflammatory infiltrates in PM and DM. An immunohistochemical analysis was performed using monoclonal antibodies to STAT1 in 57 muscle biopsies from 10 patients with DM, 10 with PM, and 37 controls. The profile of STAT1 up-regulation was also investigated in cultured muscle stimulated by interferon-gamma, epidermal growth factor, platelet-derived growth factor, and interleukin-2, using semiquantitative polymerase chain reaction and Western blot. High STAT1 expression was observed in many perifascicular atrophic muscle fibers from DM patients in 10/10 biopsies. In contrast, only a few muscle fibers undergoing necrosis were STAT1 positive in 2/10 patients with PM and in 2/37 controls. STAT1 reactivity was noted in most cells of the infiltrates in DM, PM, and controls. In vitro, STAT1 was stimulated by interferon-gamma but not by the other molecules studied. These results suggest that in DM, but not in PM, there is distinctive functional local cytokine activity able to increase STAT1 expression in muscle fibers. As interferon-gamma specifically activates STAT1 in vitro, this cytokine in conjunction with ischemia is probably involved in perifascicular muscle fiber pathology in DM.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K. A new program for investigating adult human skeletal muscle grown aneurally in tissue culture. Neurology. 1975 Jan;25(1):58–67. doi: 10.1212/wnl.25.1.58. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991 Nov 21;325(21):1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Durbin J. E., Hackenmiller R., Simon M. C., Levy D. E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996 Feb 9;84(3):443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- Emslie-Smith A. M., Arahata K., Engel A. G. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol. 1989 Mar;20(3):224–231. doi: 10.1016/0046-8177(89)90128-7. [DOI] [PubMed] [Google Scholar]
- Feldman G. M., Chuang E. J., Finbloom D. S. IgG immune complexes inhibit IFN-gamma-induced transcription of the Fc gamma RI gene in human monocytes by preventing the tyrosine phosphorylation of the p91 (Stat1) transcription factor. J Immunol. 1995 Jan 1;154(1):318–325. [PubMed] [Google Scholar]
- Gimeno R., Codony-Servat J., Plana M., Rodriguez-Sanchez J. L., Juarez C. Stat1 implication in the immune response to superantigens in vivo. J Immunol. 1996 Feb 15;156(4):1378–1386. [PubMed] [Google Scholar]
- Hohlfeld R., Engel A. G. The immunobiology of muscle. Immunol Today. 1994 Jun;15(6):269–274. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Kerr I. M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995 Feb;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Ihle J. N. STATs: signal transducers and activators of transcription. Cell. 1996 Feb 9;84(3):331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- Illa I., Dinsmore S., Dalakas M. C. Immune-mediated mechanisms and immune activation of fibroblasts in the pathogenesis of eosinophilia-myalgia syndrome induced by L-tryptophan. Hum Pathol. 1993 Jul;24(7):702–709. doi: 10.1016/0046-8177(93)90005-2. [DOI] [PubMed] [Google Scholar]
- Illa I., Leon-Monzon M., Dalakas M. C. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol. 1992 Jan;31(1):46–52. doi: 10.1002/ana.410310109. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagoo A. S., Lagoo-Deenadayalan S., Lorenz H. M., Byrne J., Barber W. H., Hardy K. J. IL-2, IL-4, and IFN-gamma gene expression versus secretion in superantigen-activated T cells. Distinct requirement for costimulatory signals through adhesion molecules. J Immunol. 1994 Feb 15;152(4):1641–1652. [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Larner A. C., Petricoin E. F., Nakagawa Y., Finbloom D. S. IL-4 attenuates the transcriptional activation of both IFN-alpha and IFN-gamma-induced cellular gene expression in monocytes and monocytic cell lines. J Immunol. 1993 Mar 1;150(5):1944–1950. [PubMed] [Google Scholar]
- Lundberg I., Brengman J. M., Engel A. G. Analysis of cytokine expression in muscle in inflammatory myopathies, Duchenne dystrophy, and non-weak controls. J Neuroimmunol. 1995 Dec;63(1):9–16. doi: 10.1016/0165-5728(95)00122-0. [DOI] [PubMed] [Google Scholar]
- Meraz M. A., White J. M., Sheehan K. C., Bach E. A., Rodig S. J., Dighe A. S., Kaplan D. H., Riley J. K., Greenlund A. C., Campbell D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996 Feb 9;84(3):431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- Mui A. L., Wakao H., O'Farrell A. M., Harada N., Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995 Mar 15;14(6):1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Seder R. A. Lymphocyte responses and cytokines. Cell. 1994 Jan 28;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Pine R., Canova A., Schindler C. Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFN alpha and IFN gamma, and is likely to autoregulate the p91 gene. EMBO J. 1994 Jan 1;13(1):158–167. doi: 10.1002/j.1460-2075.1994.tb06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Tews D. S., Goebel H. H. Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol. 1996 Mar;55(3):342–347. doi: 10.1097/00005072-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]







