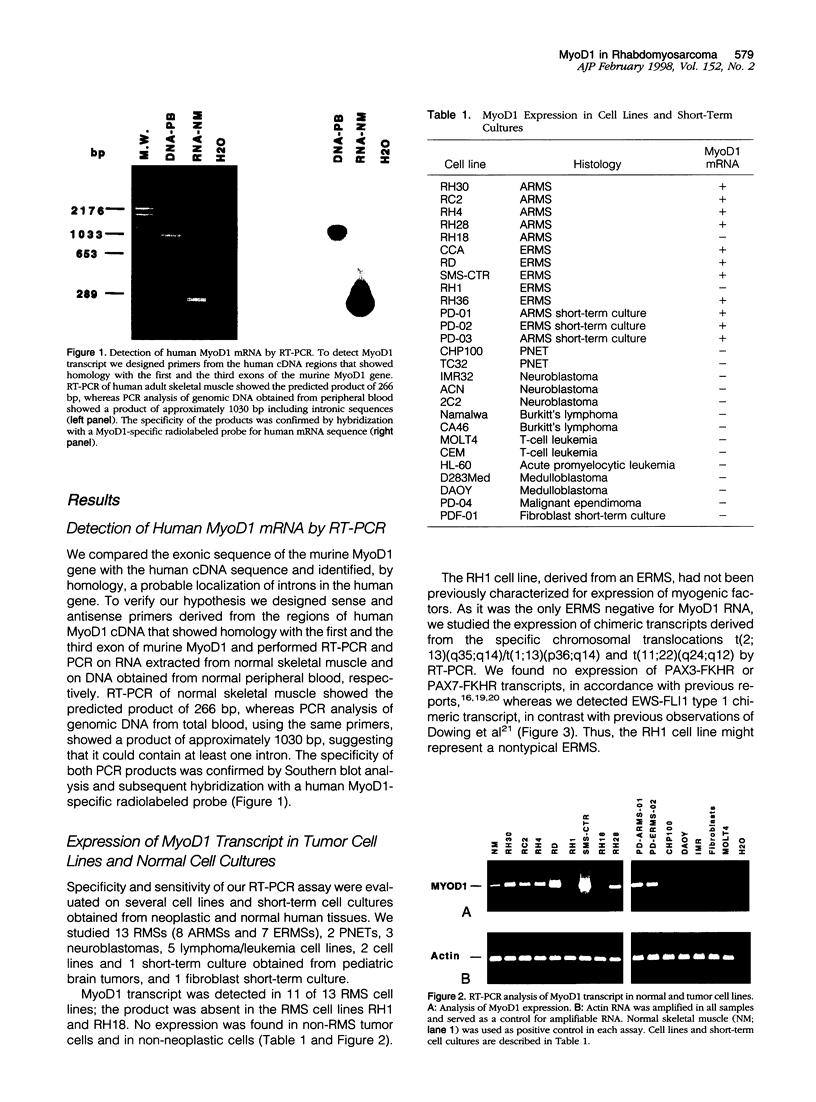
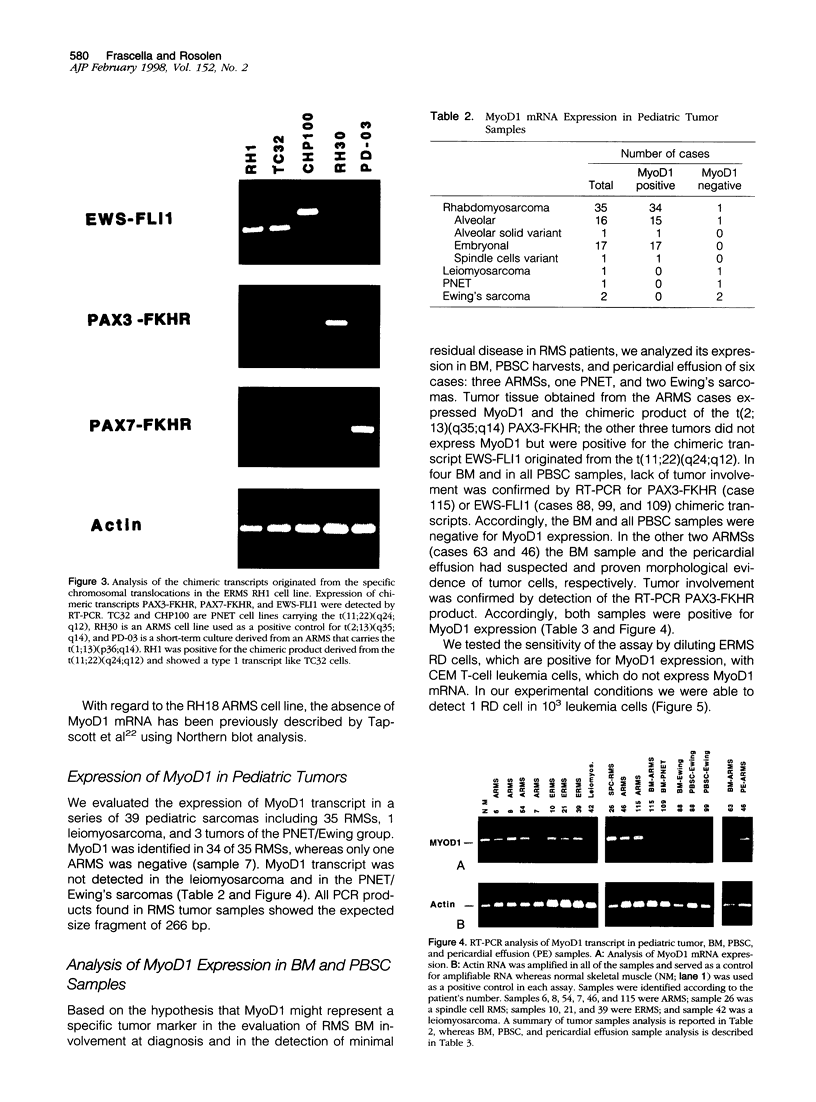
Abstract
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma of childhood. Diagnosis of RMS can be difficult when it appears as a small round-cell tumor without evidence of differentiation. Recently, a set of regulatory proteins expressed during skeletal muscle development has been described. Among them, MyoD1 has been detected by Northern blot and immunohistochemical analyses in normal skeletal muscle and RMS. Given the relevance of this marker in the diagnosis of RMS, we developed an assay to evaluate the expression of MyoD1 mRNA in small tissue specimens by reverse transcription polymerase chain reaction. Specificity and sensitivity of the assay was determined in a series of 25 tumor cell lines and 39 pediatric tumor samples, including 35 RMSs. Subsequently, we studied the expression of MyoD1 in bone marrow and peripheral blood stem cell specimens. We detected the MyoD1 transcript in normal skeletal muscle and in almost all RMSs, whereas no expression was found in non-RMS samples or in normal hematopoietic tissues. This assay showed high sensitivity and specificity, and it could be a useful molecular tool for the diagnosis of RMS within small roundcell tumors of childhood and for the detection of minimal bone marrow and peripheral blood stem cell involvement in children with RMS, regardless of the histological subtype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biegel J. A., Nycum L. M., Valentine V., Barr F. G., Shapiro D. N. Detection of the t(2;13)(q35;q14) and PAX3-FKHR fusion in alveolar rhabdomyosarcoma by fluorescence in situ hybridization. Genes Chromosomes Cancer. 1995 Mar;12(3):186–192. doi: 10.1002/gcc.2870120305. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Chen J., Gonzales F., Jones P. A., Fusenig N. E. Progressive stages of "transdifferentiation" from epidermal to mesenchymal phenotype induced by MyoD1 transfection, 5-aza-2'-deoxycytidine treatment, and selection for reduced cell attachment in the human keratinocyte line HaCaT. J Cell Biol. 1992 Mar;116(5):1257–1271. doi: 10.1083/jcb.116.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Bober E., Buschhausen-Denker G., Kohtz S., Grzeschik K. H., Arnold H. H., Kotz S. Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 1989 Dec 1;8(12):3617–3625. doi: 10.1002/j.1460-2075.1989.tb08535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J., Rocques P. J., Braun T., Bober E., Arnold H. H., Fisher C., Fletcher C., Brown K., Gusterson B. A., Carter R. L. Expression of members of the myf gene family in human rhabdomyosarcomas. Br J Cancer. 1991 Dec;64(6):1039–1042. doi: 10.1038/bjc.1991.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., D'Cruz C. M., Lovell M. A., Biegel J. A., Barr F. G. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res. 1994 Jun 1;54(11):2869–2872. [PubMed] [Google Scholar]
- Delattre O., Zucman J., Plougastel B., Desmaze C., Melot T., Peter M., Kovar H., Joubert I., de Jong P., Rouleau G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992 Sep 10;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Dias P., Parham D. M., Shapiro D. N., Tapscott S. J., Houghton P. J. Monoclonal antibodies to the myogenic regulatory protein MyoD1: epitope mapping and diagnostic utility. Cancer Res. 1992 Dec 1;52(23):6431–6439. [PubMed] [Google Scholar]
- Dias P., Parham D. M., Shapiro D. N., Webber B. L., Houghton P. J. Myogenic regulatory protein (MyoD1) expression in childhood solid tumors: diagnostic utility in rhabdomyosarcoma. Am J Pathol. 1990 Dec;137(6):1283–1291. [PMC free article] [PubMed] [Google Scholar]
- Engel M. E., Mouton S. C., Emms M. Paediatric rhabdomyosarcoma: MyoD1 demonstration in routinely processed tissue sections using wet heat pretreatment (pressure cooking) for antigen retrieval. J Clin Pathol. 1997 Jan;50(1):37–39. doi: 10.1136/jcp.50.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili N., Davis R. J., Fredericks W. J., Mukhopadhyay S., Rauscher F. J., 3rd, Emanuel B. S., Rovera G., Barr F. G. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993 Nov;5(3):230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- Pappo A. S., Shapiro D. N., Crist W. M., Maurer H. M. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995 Aug;13(8):2123–2139. doi: 10.1200/JCO.1995.13.8.2123. [DOI] [PubMed] [Google Scholar]
- Raney R. B., Jr, Tefft M., Maurer H. M., Ragab A. H., Hays D. M., Soule E. H., Foulkes M. A., Gehan E. A. Disease patterns and survival rate in children with metastatic soft-tissue sarcoma. A report from the Intergroup Rhabdomyosarcoma Study (IRS)-I. Cancer. 1988 Oct 1;62(7):1257–1266. doi: 10.1002/1097-0142(19881001)62:7<1257::aid-cncr2820620703>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Scrable H. J., Johnson D. K., Rinchik E. M., Cavenee W. K. Rhabdomyosarcoma-associated locus and MYOD1 are syntenic but separate loci on the short arm of human chromosome 11. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2182–2186. doi: 10.1073/pnas.87.6.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrable H., Witte D., Shimada H., Seemayer T., Sheng W. W., Soukup S., Koufos A., Houghton P., Lampkin B., Cavenee W. Molecular differential pathology of rhabdomyosarcoma. Genes Chromosomes Cancer. 1989 Sep;1(1):23–35. doi: 10.1002/gcc.2870010106. [DOI] [PubMed] [Google Scholar]
- Shapiro D. N., Sublett J. E., Li B., Downing J. R., Naeve C. W. Fusion of PAX3 to a member of the forkhead family of transcription factors in human alveolar rhabdomyosarcoma. Cancer Res. 1993 Nov 1;53(21):5108–5112. [PubMed] [Google Scholar]
- Sreekantaiah C., Ladanyi M., Rodriguez E., Chaganti R. S. Chromosomal aberrations in soft tissue tumors. Relevance to diagnosis, classification, and molecular mechanisms. Am J Pathol. 1994 Jun;144(6):1121–1134. [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Thayer M. J., Weintraub H. Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science. 1993 Mar 5;259(5100):1450–1453. doi: 10.1126/science.8383879. [DOI] [PubMed] [Google Scholar]
- Tonin P. N., Scrable H., Shimada H., Cavenee W. K. Muscle-specific gene expression in rhabdomyosarcomas and stages of human fetal skeletal muscle development. Cancer Res. 1991 Oct 1;51(19):5100–5106. [PubMed] [Google Scholar]
- Wang N. P., Marx J., McNutt M. A., Rutledge J. C., Gown A. M. Expression of myogenic regulatory proteins (myogenin and MyoD1) in small blue round cell tumors of childhood. Am J Pathol. 1995 Dec;147(6):1799–1810. [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993 Dec 31;75(7):1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Zingg J. M., Alva G. P., Jost J. P. Characterisation of a genomic clone covering the structural mouse MyoD1 gene and its promoter region. Nucleic Acids Res. 1991 Dec 11;19(23):6433–6439. doi: 10.1093/nar/19.23.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]