Abstract
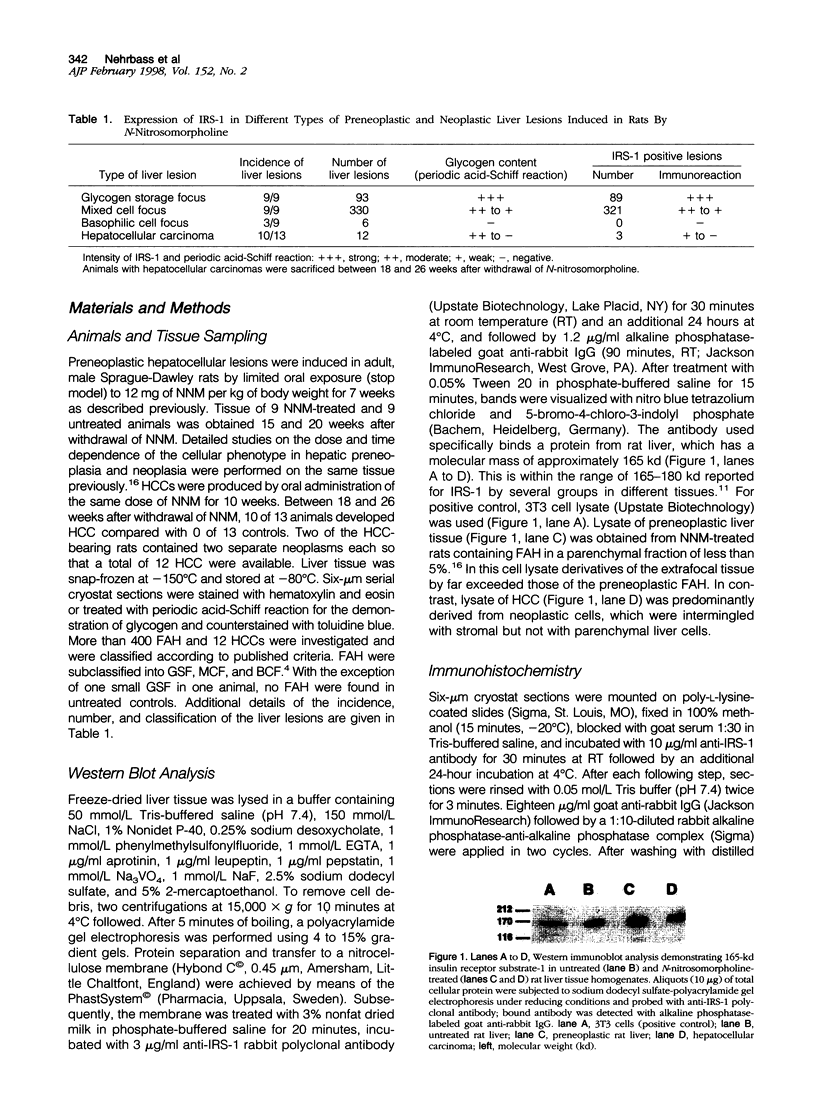
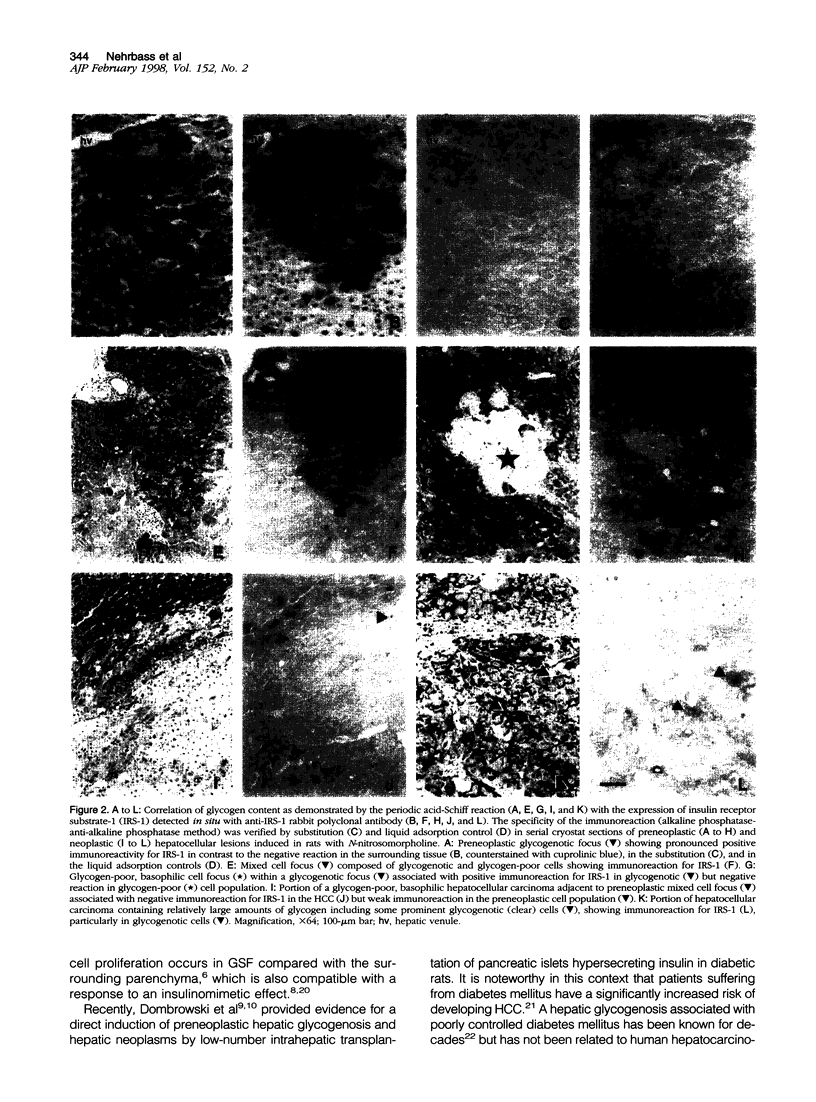
Insulin receptor substrate-1 (IRS-1) is a multisite docking protein occupying a central position in signaling cascades stimulated by a number of growth factors including insulin. Using Western blotting and immunohistochemistry, we investigated the expression of IRS-1 in more than 400 preneoplastic foci of altered hepatocytes and in 12 hepatocellular carcinomas induced in rats by oral administration of N-nitrosomorpholine. In both N-nitrosomorpholine-treated and untreated rat livers, IRS-1 was demonstrable by Western blotting, but with the exception of a few single hepatocytes it was not detectable in the normal parenchyma by immunohistochemistry. In contrast, immunohistochemistry revealed that IRS-1 was strongly expressed in the majority of foci of altered hepatocytes particularly in approximately 97% of the clear/acidophilic and mixed cell foci showing excessive storage of glycogen (glycogenosis). In glycogen-poor basophilic foci of altered hepatocytes and hepatocellular carcinomas, IRS-1 was not detected by immunohistochemistry, but a weak expression was observed in small subpopulations of three hepatocellular carcinomas containing remnants of glycogen. These results indicate that the focal overexpression of IRS-1 is an early event in hepatocarcinogenesis, which is closely correlated with preneoplastic hepatic glycogenosis. During progression from glycogenotic foci to hepatocellular carcinomas, IRS-1-overexpression is gradually down-regulated, and this late event is associated with a fundamental metabolic shift leading to the malignant neoplastic phenotype.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adami H. O., Chow W. H., Nyrén O., Berne C., Linet M. S., Ekbom A., Wolk A., McLaughlin J. K., Fraumeni J. F., Jr Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996 Oct 16;88(20):1472–1477. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- Bannasch P., Hacker H. J., Klimek F., Mayer D. Hepatocellular glycogenosis and related pattern of enzymatic changes during hepatocarcinogenesis. Adv Enzyme Regul. 1984;22:97–121. doi: 10.1016/0065-2571(84)90010-4. [DOI] [PubMed] [Google Scholar]
- Bannasch P., Klimek F., Mayer D. Early bioenergetic changes in hepatocarcinogenesis: preneoplastic phenotypes mimic responses to insulin and thyroid hormone. J Bioenerg Biomembr. 1997 Aug;29(4):303–313. doi: 10.1023/a:1022438528634. [DOI] [PubMed] [Google Scholar]
- Bannasch P., Mayer D., Hacker H. J. Hepatocellular glycogenosis and hepatocarcinogenesis. Biochim Biophys Acta. 1980 May 6;605(2):217–245. doi: 10.1016/0304-419x(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Bannasch P. Pathogenesis of hepatocellular carcinoma: sequential cellular, molecular, and metabolic changes. Prog Liver Dis. 1996;14:161–197. [PubMed] [Google Scholar]
- Bergmann U., Funatomi H., Kornmann M., Beger H. G., Korc M. Increased expression of insulin receptor substrate-1 in human pancreatic cancer. Biochem Biophys Res Commun. 1996 Mar 27;220(3):886–890. doi: 10.1006/bbrc.1996.0500. [DOI] [PubMed] [Google Scholar]
- Bollen M., Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27(3):227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- Chatila R., West A. B. Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetes. Medicine (Baltimore) 1996 Nov;75(6):327–333. doi: 10.1097/00005792-199611000-00003. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio C., Keller S. R., Morrione A., Lienhard G. E., Baserga R., Surmacz E. Transforming potential of the insulin receptor substrate 1. Cell Growth Differ. 1995 May;6(5):557–562. [PubMed] [Google Scholar]
- Dombrowski F., Bannasch P., Pfeifer U. Hepatocellular neoplasms induced by low-number pancreatic islet transplants in streptozotocin diabetic rats. Am J Pathol. 1997 Mar;150(3):1071–1087. [PMC free article] [PubMed] [Google Scholar]
- Dombrowski F., Filsinger E., Bannasch P., Pfeifer U. Altered liver acini induced in diabetic rats by portal vein islet isografts resemble preneoplastic hepatic foci in their enzymic pattern. Am J Pathol. 1996 Apr;148(4):1249–1256. [PMC free article] [PubMed] [Google Scholar]
- Furusaka A., Nishiyama M., Ohkawa K., Yamori T., Tsuruo T., Yonezawa K., Kasuga M., Hayashi S., Tanaka T. Expression of insulin receptor substrate-1 in hepatocytes: an investigation using monoclonal antibodies. Cancer Lett. 1994 Aug 29;84(1):85–92. doi: 10.1016/0304-3835(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Zhang Y., Kaufman D. G. Association between expression of transforming growth factor-alpha and progression of hepatocellular foci to neoplasms. Carcinogenesis. 1992 Aug;13(8):1481–1483. doi: 10.1093/carcin/13.8.1481. [DOI] [PubMed] [Google Scholar]
- Klimek F., Bannasch P. Isoenzyme shift from glucokinase to hexokinase is not an early but a late event in hepatocarcinogenesis. Carcinogenesis. 1993 Sep;14(9):1857–1861. doi: 10.1093/carcin/14.9.1857. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Jr, White M. F. Insulin signal transduction and the IRS proteins. Annu Rev Pharmacol Toxicol. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- Nishiyama M., Wands J. R. Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1992 Feb 28;183(1):280–285. doi: 10.1016/0006-291x(92)91640-c. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Zhang X. F., Nishiyama M., Avruch J., Wands J. R. Expression and phosphorylation of insulin receptor substrate 1 during rat liver regeneration. J Biol Chem. 1993 Feb 25;268(6):3805–3808. [PubMed] [Google Scholar]
- Schirmacher P., Rogler C. E., Dienes H. P. Current pathogenetic and molecular concepts in viral liver carcinogenesis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(2):71–89. doi: 10.1007/BF02899246. [DOI] [PubMed] [Google Scholar]
- Su Q., Benner A., Hofmann W. J., Otto G., Pichlmayr R., Bannasch P. Human hepatic preneoplasia: phenotypes and proliferation kinetics of foci and nodules of altered hepatocytes and their relationship to liver cell dysplasia. Virchows Arch. 1997 Dec;431(6):391–406. doi: 10.1007/s004280050116. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Wands J. R. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J Clin Invest. 1996 Nov 1;98(9):2100–2108. doi: 10.1172/JCI119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Wands J. R. Insulin receptor substrate 1 overexpression in human hepatocellular carcinoma cells prevents transforming growth factor beta1-induced apoptosis. Cancer Res. 1996 Aug 1;56(15):3391–3394. [PubMed] [Google Scholar]
- Weber E., Bannasch P. Dose and time dependence of the cellular phenotype in rat hepatic preneoplasia and neoplasia induced in stop experiments by oral exposure to N-nitrosomorpholine. Carcinogenesis. 1994 Jun;15(6):1227–1234. doi: 10.1093/carcin/15.6.1227. [DOI] [PubMed] [Google Scholar]
- Zerban H., Radig S., Kopp-Schneider A., Bannasch P. Cell proliferation and cell death (apoptosis) in hepatic preneoplasia and neoplasia are closely related to phenotypic cellular diversity and instability. Carcinogenesis. 1994 Nov;15(11):2467–2473. doi: 10.1093/carcin/15.11.2467. [DOI] [PubMed] [Google Scholar]




