Abstract
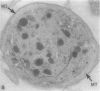
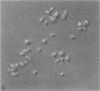
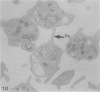
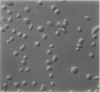
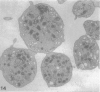
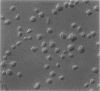
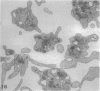
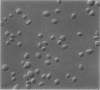
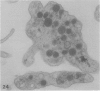
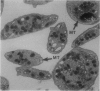
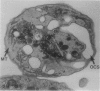
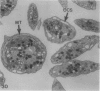
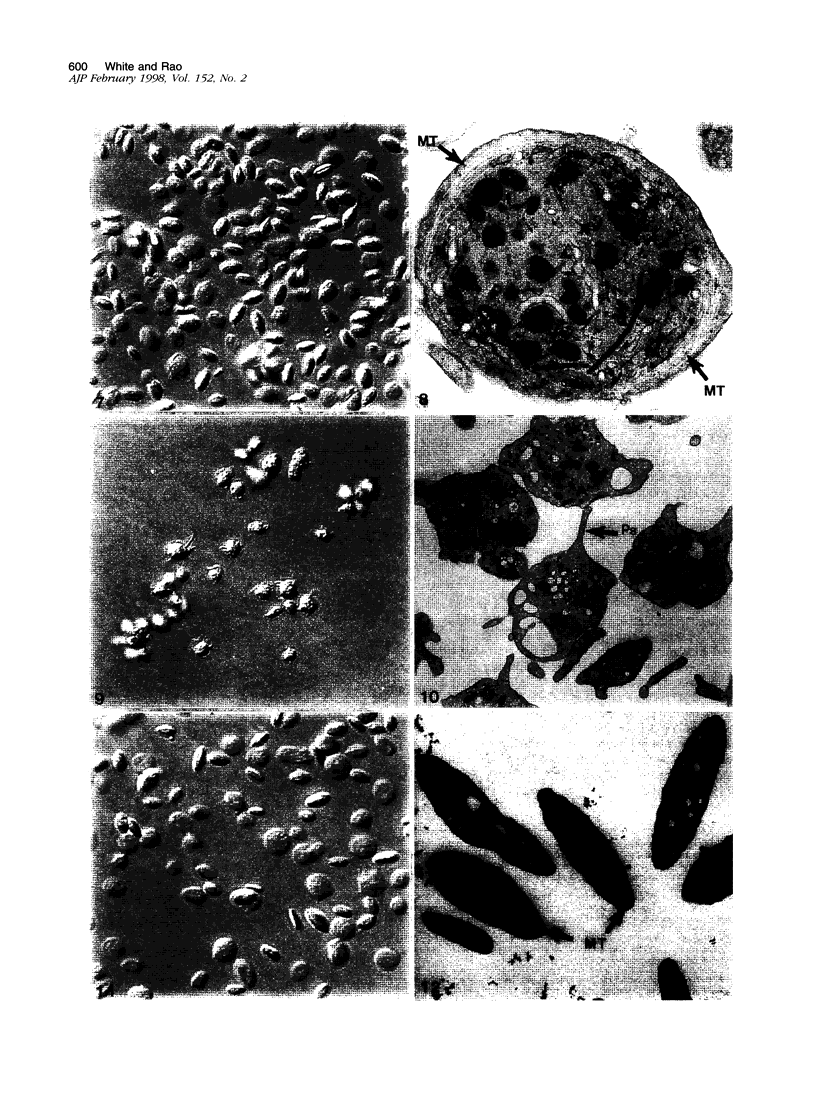
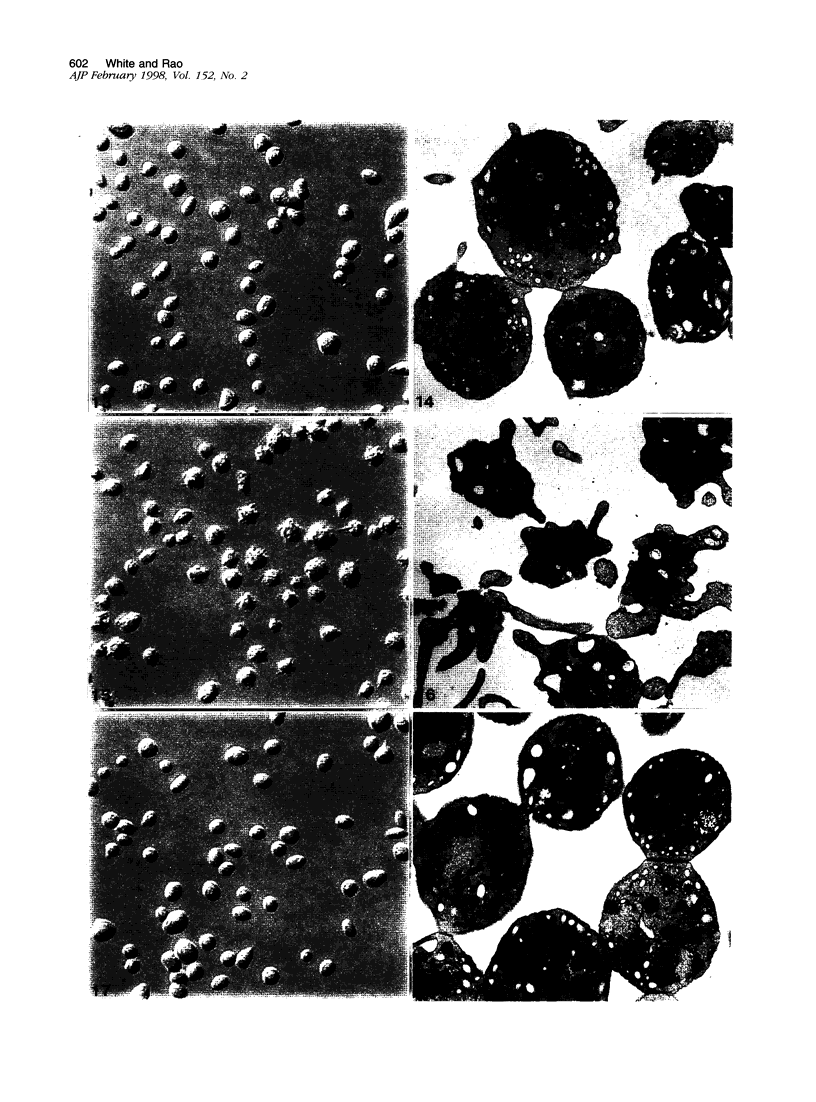
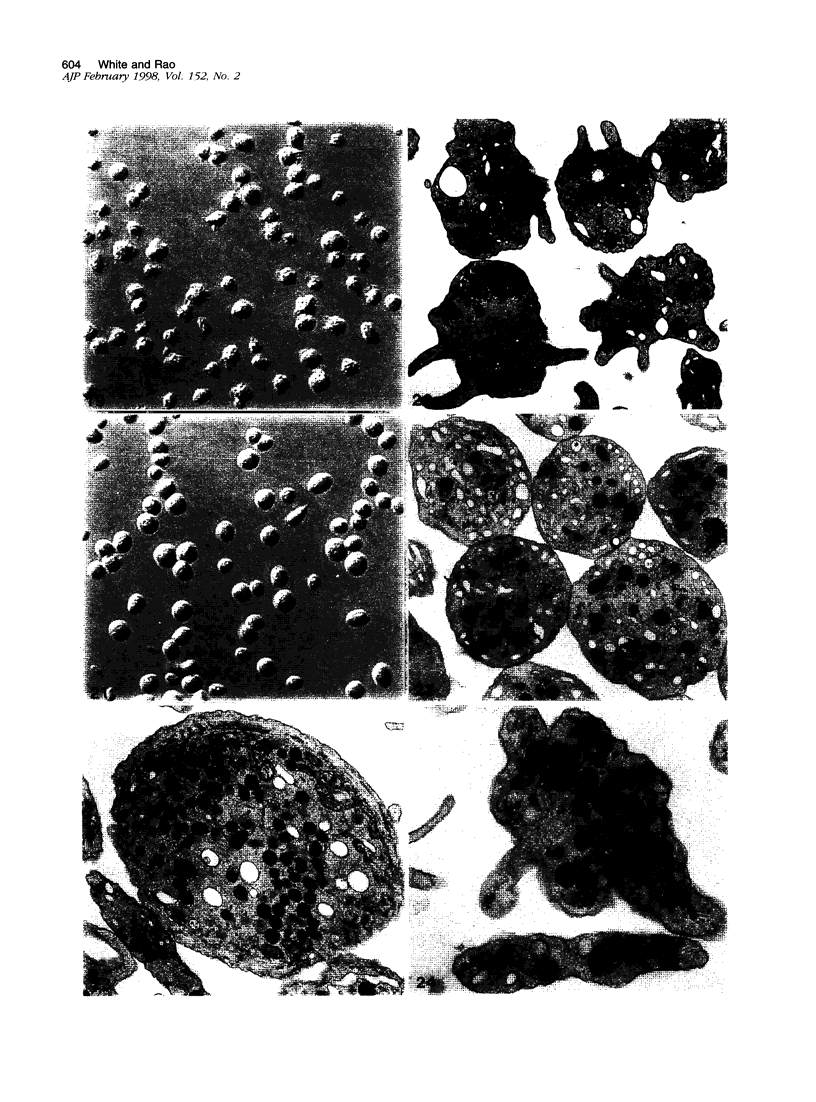
The discoid form of blood platelets is important to their function in hemostasis. Recent studies have suggested that the spectrin-rich surface membrane cytoskeleton and the cytoplasmic, actin-rich cytoskeleton are responsible for discoid shape, shape change, and recovery after activation or chilling. Earlier studies had suggested that circumferential coils of microtubules supported the disc shape of resting platelets and that their repositioning or reassembly restored disc shape after exposure to low temperature. The present study has used the chilling-rewarming model, together with microtubule stabilizing (taxol) and disassembling (vincristine) agents to retest the relative importance of the surface membrane cytoskeleton and circumferential microtubules in platelet discoid shape and its restoration. Washed platelet samples were rested at 37 degrees C and chilled to 4 degrees C; chilled and rewarmed to 37 degrees C for 60 minutes; or chilled, rewarmed, and exposed to the same cycle in the presence or absence of vincristine or taxol and fixed for study by disseminated interference phase contrast microscopy and electron microscopy. Rhodamine-phalloidin and flow cytometry were used to measure changes in actin filament assembly. Chilling caused loss of disc shape, pseudopod extension, disassembly of microtubule coils, and assembly of new actin filaments. Rewarming resulted in restoration of disc shape, pseudopod retraction, disassembly of new actin filaments, and reassembly of circumferential microtubule coils. Vincristine converted discoid platelets to rounded cells that extended pseudopods when chilled and retracted them when rewarmed, leaving spheres that could undergo the same sequence of changes when chilled and rewarmed again. Taxol prevented cold-induced disassembly of microtubules and limited pseudopod formation. Rewarming caused retraction of pseudopods on taxol-treated, discoid cells. Cytochalasin B, an agent that blocks new actin filament assembly, alone or in combination with taxol, inhibited the cold-induced shape change but not dilation of the open canalicular system. Rewarming eliminated open canalicular system dilation and restored lentiform appearance. The results indicate that microtubule coils are the major structural elements responsible for disc shape and its restoration after submaximal stimulation or rewarming of chilled platelets.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antin P. B., Forry-Schaudies S., Friedman T. M., Tapscott S. J., Holtzer H. Taxol induces postmitotic myoblasts to assemble interdigitating microtubule-myosin arrays that exclude actin filaments. J Cell Biol. 1981 Aug;90(2):300–308. doi: 10.1083/jcb.90.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke O. Further studies on microtubules. A marginal bundle in human and rat thrombocytes. J Ultrastruct Res. 1965 Dec;13(5):469–477. doi: 10.1016/s0022-5320(65)90009-2. [DOI] [PubMed] [Google Scholar]
- Behnke O. Incomplete microtubules observed in mammalian blood platelets during microtubule polymerization. J Cell Biol. 1967 Aug;34(2):697–701. doi: 10.1083/jcb.34.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis M., Breton-Gorius J. Les microtubules et les fibrilles dans les plaquettes étalées. Nouv Rev Fr Hematol. 1965 Jul-Aug;5(4):657–662. [PubMed] [Google Scholar]
- Carlsson L., Markey F., Blikstad I., Persson T., Lindberg U. Reorganization of actin in platelets stimulated by thrombin as measured by the DNase I inhibition assay. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6376–6380. doi: 10.1073/pnas.76.12.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Boyles J. K., Reynolds C. C., Phillips D. R. Actin filament content and organization in unstimulated platelets. J Cell Biol. 1984 Jun;98(6):1985–1991. doi: 10.1083/jcb.98.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Inhibition of actin polymerization in blood platelets by cytochalasins. Nature. 1981 Aug 13;292(5824):650–652. doi: 10.1038/292650a0. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouse L. H., Rao G. H., Weiss D. J., Perman V., White J. G. Surface-activated bovine platelets do not spread, they unfold. Am J Pathol. 1990 Feb;136(2):399–408. [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., DeSisto M. The cytoskeleton of the resting human blood platelet: structure of the membrane skeleton and its attachment to actin filaments. J Cell Biol. 1991 Feb;112(3):407–425. doi: 10.1083/jcb.112.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992 Sep;118(6):1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon G. B., Taylor D. A. Microtubules in hamster platelets. J Cell Biol. 1965 Aug;26(2):673–676. doi: 10.1083/jcb.26.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M. Temperature cycling preserves platelet shape and enhances in vitro test scores during storage at 4 degrees. J Lab Clin Med. 1978 Dec;92(6):971–982. [PubMed] [Google Scholar]
- Oda A., Daley J. F., Cabral C., Kang J. H., Smith M., Salzman E. W. Heterogeneity in filamentous actin content among individual human blood platelets. Blood. 1992 Feb 15;79(4):920–927. [PubMed] [Google Scholar]
- Pribluda V., Rotman A. Dynamics of membrane-cytoskeleton interactions in activated blood platelets. Biochemistry. 1982 Jun 8;21(12):2825–2832. doi: 10.1021/bi00541a003. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. M., 2nd, Burris S. M., Weiss D. J., White J. G. Comparison of bovine and human platelet deformability, using micropipette elastimetry. Am J Vet Res. 1989 Jan;50(1):34–38. [PubMed] [Google Scholar]
- Takeuchi K., Kuroda K., Ishigami M., Nakamura T. Actin cytoskeleton of resting bovine platelets. Exp Cell Res. 1990 Feb;186(2):374–380. doi: 10.1016/0014-4827(90)90319-6. [DOI] [PubMed] [Google Scholar]
- Wani M. C., Taylor H. L., Wall M. E., Coggon P., McPhail A. T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971 May 5;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- White J. G. An overview of platelet structural physiology. Scanning Microsc. 1987 Dec;1(4):1677–1700. [PubMed] [Google Scholar]
- White J. G. Effects of colchicine and Vinca alkaloids on human platelets. I. Influence on platelet microtubules and contractile function. Am J Pathol. 1968 Aug;53(2):281–291. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- White J. G. Influence of microtubule stabilization on platelet physiology. Trans Assoc Am Physicians. 1982;95:264–271. [PubMed] [Google Scholar]
- White J. G. Influence of taxol on the response of platelets to chilling. Am J Pathol. 1982 Aug;108(2):184–195. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Interaction of membrane systems in blood platelets. Am J Pathol. 1972 Feb;66(2):295–312. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Krivit W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood. 1967 Nov;30(5):625–635. [PubMed] [Google Scholar]
- White J. G., Krumwiede M. Influence of cytochalasin B on the shape change induced in platelets by cold. Blood. 1973 Jun;41(6):823–832. [PubMed] [Google Scholar]
- White J. G., Krumwiede M., Sauk J. J. Microtubule reassembly in surface-activated platelets. Blood. 1985 Jun;65(6):1494–1503. [PubMed] [Google Scholar]
- White J. G., Krumwiede M., Sauk J. J. Microtubule reassembly in surface-activated platelets. Blood. 1985 Jun;65(6):1494–1503. [PubMed] [Google Scholar]
- White J. G. Platelet structural physiology: the ultrastructure of adhesion, secretion, and aggregation in arterial thrombosis. Cardiovasc Clin. 1987;18(1):13–33. [PubMed] [Google Scholar]
- White J. G., Sauk J. J. Microtubule coils in spread blood platelets. Blood. 1984 Aug;64(2):470–478. [PubMed] [Google Scholar]
- White J. G. Ultrastructural physiology of platelets with randomly dispersed rather than circumferential band microtubules. Am J Pathol. 1983 Jan;110(1):55–63. [PMC free article] [PubMed] [Google Scholar]
- Winokur R., Hartwig J. H. Mechanism of shape change in chilled human platelets. Blood. 1995 Apr 1;85(7):1796–1804. [PubMed] [Google Scholar]