Abstract
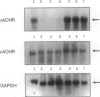
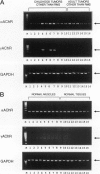
The fetal nicotinic acetylcholine receptor (AChR) of muscle is an oligomeric membrane protein with subunit composition alpha2betadeltagamma. After birth, the adult form, in which an epsilon-subunit replaces the gamma-subunit, predominates, and expression of the fetal form is limited to thymic myoid cells, extraocular muscles, and denervated striated muscle. We looked for expression of AChR in rhabdomyosarcomas and other childhood tumors by reverse transcription polymerase chain reaction and immunohistochemistry. mRNA for the AChR gamma-subunit was detected in all embryonal and alveolar rhabdomyosarcomas tested (n = 16) and in some tumors with a rhabdomyomatous component (n = 2) but not in other nonrhabdomyomatous tumors of childhood and adults (n = 45). The fetal form of the AChR was detected immunohistochemically in five of eight embryonal and four of eight alveolar rhabdomyosarcomas and in two Wilms' tumors with a rhabdomyomatous component but not in other tumors or in normal muscle. We conclude that reverse transcription polymerase chain reaction for AChR gamma-subunit could be useful for the diagnosis of rhabdomyosarcoma of childhood and for the detection of micrometastases and minimal residual disease. In addition, the fetal AChR protein is the first extracellular tumor marker that can distinguish rhabdomyosarcomas from nonrhabdomyomatous tumors and from normal muscle. Our findings, therefore, imply that the fetal AChR may be a target for in vivo imaging and, as AChR internalization and degradation is increased by antibody-induced cross-linking, may also provide a sensitive and specific target for immunotherapeutic strategies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeson D., Brydson M., Betty M., Jeremiah S., Povey S., Vincent A., Newsom-Davis J. Primary structure of the human muscle acetylcholine receptor. cDNA cloning of the gamma and epsilon subunits. Eur J Biochem. 1993 Jul 15;215(2):229–238. doi: 10.1111/j.1432-1033.1993.tb18027.x. [DOI] [PubMed] [Google Scholar]
- Beeson D., Brydson M., Newsom-Davis J. Nucleotide sequence of human muscle acetylcholine receptor beta-subunit. Nucleic Acids Res. 1989 Jun 12;17(11):4391–4391. doi: 10.1093/nar/17.11.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegel J. A., Meek R. S., Parmiter A. H., Conard K., Emanuel B. S. Chromosomal translocation t(1;13)(p36;q14) in a case of rhabdomyosarcoma. Genes Chromosomes Cancer. 1991 Nov;3(6):483–484. doi: 10.1002/gcc.2870030612. [DOI] [PubMed] [Google Scholar]
- Carter R. L., McCarthy K. P., Machin L. G., Jameson C. F., Philp E. R., Pinkerton C. R. Expression of desmin and myoglobin in rhabdomyosarcomas and in developing skeletal muscle. Histopathology. 1989 Dec;15(6):585–595. doi: 10.1111/j.1365-2559.1989.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Corrado K., Rafael J. A., Cox G. A., Hauser M., Lumeng C. Interactions between dystrophin and the sarcolemma membrane. Soc Gen Physiol Ser. 1997;52:19–29. [PubMed] [Google Scholar]
- Changeux J. P. Functional organisation of the nicotinic acetylcholine receptor. C R Acad Sci III. 1992;314(9 Suppl):89–94. [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dias P., Parham D. M., Shapiro D. N., Webber B. L., Houghton P. J. Myogenic regulatory protein (MyoD1) expression in childhood solid tumors: diagnostic utility in rhabdomyosarcoma. Am J Pathol. 1990 Dec;137(6):1283–1291. [PMC free article] [PubMed] [Google Scholar]
- Diller L. Rhabdomyosarcoma and other soft tissue sarcomas of childhood. Curr Opin Oncol. 1992 Aug;4(4):689–695. doi: 10.1097/00001622-199208000-00014. [DOI] [PubMed] [Google Scholar]
- Douglass E. C., Rowe S. T., Valentine M., Parham D. M., Berkow R., Bowman W. P., Maurer H. M. Variant translocations of chromosome 13 in alveolar rhabdomyosarcoma. Genes Chromosomes Cancer. 1991 Nov;3(6):480–482. doi: 10.1002/gcc.2870030611. [DOI] [PubMed] [Google Scholar]
- Douglass E. C., Shapiro D. N., Valentine M., Rowe S. T., Carroll A. J., Raney R. B., Ragab A. H., Abella S. M., Parham D. M. Alveolar rhabdomyosarcoma with the t(2;13): cytogenetic findings and clinicopathologic correlations. Med Pediatr Oncol. 1993;21(2):83–87. doi: 10.1002/mpo.2950210202. [DOI] [PubMed] [Google Scholar]
- Douglass E. C., Valentine M., Etcubanas E., Parham D., Webber B. L., Houghton P. J., Houghton J. A., Green A. A. A specific chromosomal abnormality in rhabdomyosarcoma. Cytogenet Cell Genet. 1987;45(3-4):148–155. doi: 10.1159/000132446. [DOI] [PubMed] [Google Scholar]
- Galili N., Davis R. J., Fredericks W. J., Mukhopadhyay S., Rauscher F. J., 3rd, Emanuel B. S., Rovera G., Barr F. G. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993 Nov;5(3):230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- Hara H., Hayashi K., Ohta K., Itoh N., Ohta M. Nicotinic acetylcholine receptor mRNAs in myasthenic thymuses: association with intrathymic pathogenesis of myasthenia gravis. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1269–1275. doi: 10.1006/bbrc.1993.1960. [DOI] [PubMed] [Google Scholar]
- Heidenreich F., Vincent A., Roberts A., Newsom-Davis J. Epitopes on human acetylcholine receptor defined by monoclonal antibodies and myasthenia gravis sera. Autoimmunity. 1988;1(4):285–297. doi: 10.3109/08916938809010682. [DOI] [PubMed] [Google Scholar]
- Hesselmans L. F., Jennekens F. G., Van den Oord C. J., Veldman H., Vincent A. Development of innervation of skeletal muscle fibers in man: relation to acetylcholine receptors. Anat Rec. 1993 Jul;236(3):553–562. doi: 10.1002/ar.1092360315. [DOI] [PubMed] [Google Scholar]
- Jong A. S., van Vark M., Albus-Lutter C. E., van Raamsdonk W., Voûte P. A. Myosin and myoglobin as tumor markers in the diagnosis of rhabdomyosarcoma. A comparative study. Am J Surg Pathol. 1984 Jul;8(7):521–528. doi: 10.1097/00000478-198407000-00004. [DOI] [PubMed] [Google Scholar]
- Kaminski H. J., Kusner L. L., Block C. H. Expression of acetylcholine receptor isoforms at extraocular muscle endplates. Invest Ophthalmol Vis Sci. 1996 Feb;37(2):345–351. [PubMed] [Google Scholar]
- Kirchner T., Geuder K. I., Marx A., Müller-Hermelink H. K. Nikotinische Azetylcholin-Rezeptoren in Tumoren mit rhabdomyomatöser Differenzierung. Immunhistochemischer und molekulargenetischer Nachweis. Verh Dtsch Ges Pathol. 1990;74:409–414. [PubMed] [Google Scholar]
- Kuncl R. W., Drachman D. B., Adams R., Lehar M. 3-Deazaadenosine: a therapeutic strategy for myasthenia gravis by decreasing the endocytosis of acetylcholine receptors. J Pharmacol Exp Ther. 1993 Nov;267(2):582–589. [PubMed] [Google Scholar]
- Luther M. A., Schoepfer R., Whiting P., Casey B., Blatt Y., Montal M. S., Montal M., Linstrom J. A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci. 1989 Mar;9(3):1082–1096. doi: 10.1523/JNEUROSCI.09-03-01082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx A., Kirchner T., Hoppe F., O'Connor R., Schalke B., Tzartos S., Müller-Hermelink H. K. Proteins with epitopes of the acetylcholine receptor in epithelial cell cultures of thymomas in myasthenia gravis. Am J Pathol. 1989 Apr;134(4):865–877. [PMC free article] [PubMed] [Google Scholar]
- Miettinen M. Antibody specific to muscle actins in the diagnosis and classification of soft tissue tumors. Am J Pathol. 1988 Jan;130(1):205–215. [PMC free article] [PubMed] [Google Scholar]
- Missias A. C., Chu G. C., Klocke B. J., Sanes J. R., Merlie J. P. Maturation of the acetylcholine receptor in skeletal muscle: regulation of the AChR gamma-to-epsilon switch. Dev Biol. 1996 Oct 10;179(1):223–238. doi: 10.1006/dbio.1996.0253. [DOI] [PubMed] [Google Scholar]
- Newton W. A., Jr, Gehan E. A., Webber B. L., Marsden H. B., van Unnik A. J., Hamoudi A. B., Tsokos M. G., Shimada H., Harms D., Schmidt D. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer. 1995 Sep 15;76(6):1073–1085. doi: 10.1002/1097-0142(19950915)76:6<1073::aid-cncr2820760624>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Osborn M., Hill C., Altmannsberger M., Weber K. Monoclonal antibodies to titin in conjunction with antibodies to desmin separate rhabdomyosarcomas from other tumor types. Lab Invest. 1986 Jul;55(1):101–108. [PubMed] [Google Scholar]
- Pappo A. S., Shapiro D. N., Crist W. M. Rhabdomyosarcoma. Biology and treatment. Pediatr Clin North Am. 1997 Aug;44(4):953–972. doi: 10.1016/s0031-3955(05)70539-3. [DOI] [PubMed] [Google Scholar]
- Parham D. M., Webber B., Holt H., Williams W. K., Maurer H. Immunohistochemical study of childhood rhabdomyosarcomas and related neoplasms. Results of an Intergroup Rhabdomyosarcoma study project. Cancer. 1991 Jun 15;67(12):3072–3080. doi: 10.1002/1097-0142(19910615)67:12<3072::aid-cncr2820671223>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Pinto A., Paslawski D., Sarnat H. B., Parham D. M. Immunohistochemical evaluation of dystrophin expression in small round cell tumors of childhood. Mod Pathol. 1993 Nov;6(6):679–683. [PubMed] [Google Scholar]
- Rosai J., Dias P., Parham D. M., Shapiro D. N., Houghton P. MyoD1 protein expression in alveolar soft part sarcoma as confirmatory evidence of its skeletal muscle nature. Am J Surg Pathol. 1991 Oct;15(10):974–981. doi: 10.1097/00000478-199110000-00008. [DOI] [PubMed] [Google Scholar]
- Sainati L., Stella M., Montaldi A., Bolcato S., Guercini N., Bonan F., Ninfo V., Zanesco L., Basso G. Value of cytogenetics in the differential diagnosis of the small round cell tumors of childhood. Med Pediatr Oncol. 1992;20(2):130–135. doi: 10.1002/mpo.2950200208. [DOI] [PubMed] [Google Scholar]
- Schoepfer R., Luther M., Lindstrom J. The human medulloblastoma cell line TE671 expresses a muscle-like acetylcholine receptor. Cloning of the alpha-subunit cDNA. FEBS Lett. 1988 Jan 4;226(2):235–240. doi: 10.1016/0014-5793(88)81430-3. [DOI] [PubMed] [Google Scholar]
- Scrable H., Witte D., Shimada H., Seemayer T., Sheng W. W., Soukup S., Koufos A., Houghton P., Lampkin B., Cavenee W. Molecular differential pathology of rhabdomyosarcoma. Genes Chromosomes Cancer. 1989 Sep;1(1):23–35. doi: 10.1002/gcc.2870010106. [DOI] [PubMed] [Google Scholar]
- Sophianos D., Tzartos S. J. Fab fragments of monoclonal antibodies protect the human acetylcholine receptor against antigenic modulation caused by myasthenic sera. J Autoimmun. 1989 Dec;2(6):777–789. doi: 10.1016/0896-8411(89)90004-8. [DOI] [PubMed] [Google Scholar]
- Stratton M. R., Reeves B. R., Cooper C. S. Misidentified cell. Nature. 1989 Jan 26;337(6205):311–312. doi: 10.1038/337311c0. [DOI] [PubMed] [Google Scholar]
- Tonin P. N., Scrable H., Shimada H., Cavenee W. K. Muscle-specific gene expression in rhabdomyosarcomas and stages of human fetal skeletal muscle development. Cancer Res. 1991 Oct 1;51(19):5100–5106. [PubMed] [Google Scholar]
- Trent J., Casper J., Meltzer P., Thompson F., Fogh J. Nonrandom chromosome alterations in rhabdomyosarcoma. Cancer Genet Cytogenet. 1985 Apr 1;16(3):189–197. doi: 10.1016/0165-4608(85)90045-7. [DOI] [PubMed] [Google Scholar]
- Turc-Carel C., Lizard-Nacol S., Justrabo E., Favrot M., Philip T., Tabone E. Consistent chromosomal translocation in alveolar rhabdomyosarcoma. Cancer Genet Cytogenet. 1986 Jan 15;19(3-4):361–362. doi: 10.1016/0165-4608(86)90069-5. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J. Myasthenia gravis studied by monoclonal antibodies to the acetylcholine receptor. In Vivo. 1988 Jan-Feb;2(1):105–110. [PubMed] [Google Scholar]
- Vincent A., Newland C., Brueton L., Beeson D., Riemersma S., Huson S. M., Newsom-Davis J. Arthrogryposis multiplex congenita with maternal autoantibodies specific for a fetal antigen. Lancet. 1995 Jul 1;346(8966):24–25. doi: 10.1016/s0140-6736(95)92652-6. [DOI] [PubMed] [Google Scholar]
- Wakkach A., Guyon T., Bruand C., Tzartos S., Cohen-Kaminsky S., Berrih-Aknin S. Expression of acetylcholine receptor genes in human thymic epithelial cells: implications for myasthenia gravis. J Immunol. 1996 Oct 15;157(8):3752–3760. [PubMed] [Google Scholar]
- Wang N. P., Marx J., McNutt M. A., Rutledge J. C., Gown A. M. Expression of myogenic regulatory proteins (myogenin and MyoD1) in small blue round cell tumors of childhood. Am J Pathol. 1995 Dec;147(6):1799–1810. [PMC free article] [PubMed] [Google Scholar]
- Wessels A., Ginjaar I. B., Moorman A. F., van Ommen G. J. Different localization of dystrophin in developing and adult human skeletal muscle. Muscle Nerve. 1991 Jan;14(1):1–7. doi: 10.1002/mus.880140102. [DOI] [PubMed] [Google Scholar]
- Whiting P. J., Vincent A., Schluep M., Newsom-Davis J. Monoclonal antibodies that distinguish between normal and denervated human acetylcholine receptor. J Neuroimmunol. 1986 May;11(3):223–235. doi: 10.1016/0165-5728(86)90006-8. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Barg B., Nishikawa Y., Sakmann B., Numa S. Differential regulation of muscle acetylcholine receptor gamma- and epsilon-subunit mRNAs. FEBS Lett. 1987 Oct 19;223(1):104–112. doi: 10.1016/0014-5793(87)80518-5. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- d'Amore E. S., Tollot M., Stracca-Pansa V., Menegon A., Meli S., Carli M., Ninfo V. Therapy associated differentiation in rhabdomyosarcomas. Mod Pathol. 1994 Jan;7(1):69–75. [PubMed] [Google Scholar]