Abstract
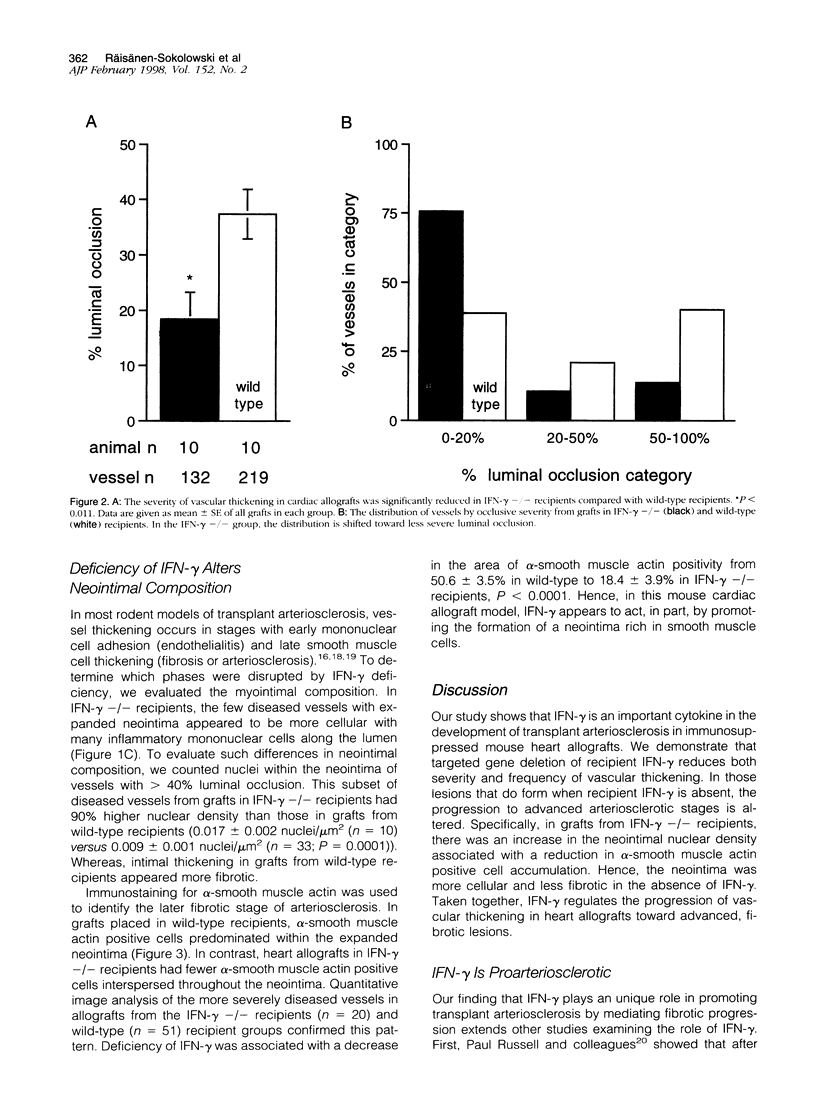
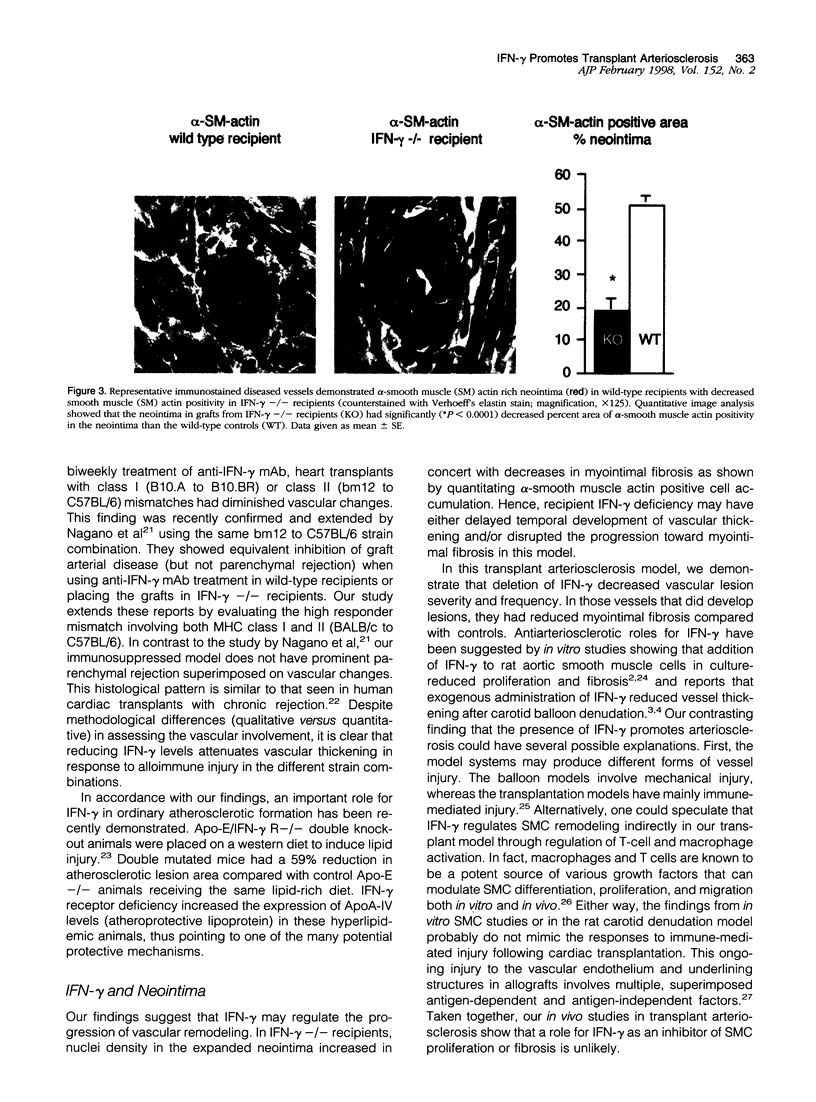
To investigate the functional role of interferon (IFN)-gamma in transplant arteriosclerosis, BALB/c hearts were transplanted in immunosuppressed C57BL/6J recipients with (n = 10) or without (n = 10) targeted IFN-gamma gene deletion. In 55-day heart allografts, IFN-gamma deficiency resulted in a significant decrease in vascular thickening. The severity of intimal thickening measured as the percentage of luminal occlusion (mean +/- SEM) in all elastin stained vessels (n = 410) decreased from 37+/-5% in wild-type recipients to 18+/-5% in IFN-gamma -/- recipients (P < 0.005). In the few diseased vessels in grafts from IFN-gamma -/- recipients, the neointima was more cellular with a 90% increase in the nuclear density. This finding correlated with a 50% reduction in fibrosis estimated by alpha-smooth muscle actin cell accumulation in the neointima. The reduction in severity and altered composition of vascular thickening in grafts from IFN-gamma -/- recipients shows that IFN-gamma contributes to arteriosclerotic development following transplantation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Tilney N. L., Collins J. J., Jr, Karnovsky M. J. Experimental graft arteriosclerosis. I. The Lewis-to-F-344 allograft model. Transplantation. 1992 May;53(5):1115–1119. [PubMed] [Google Scholar]
- Adams D. H., Wyner L. R., Karnovsky M. J. Experimental graft arteriosclerosis. II. Immunocytochemical analysis of lesion development. Transplantation. 1993 Oct;56(4):794–799. doi: 10.1097/00007890-199310000-00004. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Evan G. I., Newby A. C. Deregulated expression of the c-myc oncogene abolishes inhibition of proliferation of rat vascular smooth muscle cells by serum reduction, interferon-gamma, heparin, and cyclic nucleotide analogues and induces apoptosis. Circ Res. 1994 Mar;74(3):525–536. doi: 10.1161/01.res.74.3.525. [DOI] [PubMed] [Google Scholar]
- Castronuovo J. J., Jr, Guss S. B., Mysh D., Sawhney A., Wolff M., Gown A. M. Cytokine therapy for arterial restenosis: inhibition of neointimal hyperplasia by gamma-interferon. Cardiovasc Surg. 1995 Oct;3(5):463–468. doi: 10.1016/0967-2109(95)94442-y. [DOI] [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Dai W. J., Bartens W., Köhler G., Hufnagel M., Kopf M., Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997 Jun 1;158(11):5297–5304. [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Goes N., Urmson J., Hobart M., Halloran P. F. The unique role of interferon-gamma in the regulation of MHC expression on arterial endothelium. Transplantation. 1996 Dec 27;62(12):1889–1894. doi: 10.1097/00007890-199612270-00036. [DOI] [PubMed] [Google Scholar]
- Gupta S., Pablo A. M., Jiang X. c., Wang N., Tall A. R., Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997 Jun 1;99(11):2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J. Interferon-gamma inhibits arterial stenosis after injury. Circulation. 1991 Sep;84(3):1266–1272. doi: 10.1161/01.cir.84.3.1266. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Clowes M. M., Clowes A. W. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988 Oct;63(4):712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993 Mar 19;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Manoury-Schwartz B., Chiocchia G., Bessis N., Abehsira-Amar O., Batteux F., Muller S., Huang S., Boissier M. C., Fournier C. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997 Jun 1;158(11):5501–5506. [PubMed] [Google Scholar]
- Nagano H., Mitchell R. N., Taylor M. K., Hasegawa S., Tilney N. L., Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997 Aug 1;100(3):550–557. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Russell M. E., Hancock W. W., Akalin E., Wallace A. F., Glysing-Jensen T., Willett T. A., Sayegh M. H. Chronic cardiac rejection in the LEW to F344 rat model. Blockade of CD28-B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest. 1996 Feb 1;97(3):833–838. doi: 10.1172/JCI118483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. E., Wallace A. F., Hancock W. W., Sayegh M. H., Adams D. H., Sibinga N. E., Wyner L. R., Karnovsky M. J. Upregulation of cytokines associated with macrophage activation in the Lewis-to-F344 rat transplantation model of chronic cardiac rejection. Transplantation. 1995 Feb 27;59(4):572–578. [PubMed] [Google Scholar]
- Russell P. S., Chase C. M., Winn H. J., Colvin R. B. Coronary atherosclerosis in transplanted mouse hearts. I. Time course and immunogenetic and immunopathological considerations. Am J Pathol. 1994 Feb;144(2):260–274. [PMC free article] [PubMed] [Google Scholar]
- Russell P. S., Chase C. M., Winn H. J., Colvin R. B. Coronary atherosclerosis in transplanted mouse hearts. III. Effects of recipient treatment with a monoclonal antibody to interferon-gamma. Transplantation. 1994 May 15;57(9):1367–1371. [PubMed] [Google Scholar]
- Räisänen-Sokolowski A., Glysing-Jensen T., Mottram P. L., Russell M. E. Sustained anti-CD4/CD8 treatment blocks inflammatory activation and intimal thickening in mouse heart allografts. Arterioscler Thromb Vasc Biol. 1997 Oct;17(10):2115–2122. doi: 10.1161/01.atv.17.10.2115. [DOI] [PubMed] [Google Scholar]
- Räisänen-Sokolowski A., Mottram P. L., Glysing-Jensen T., Satoskar A., Russell M. E. Heart transplants in interferon-gamma, interleukin 4, and interleukin 10 knockout mice. Recipient environment alters graft rejection. J Clin Invest. 1997 Nov 15;100(10):2449–2456. doi: 10.1172/JCI119787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S., Konieczny B. T., Lowry R. P., Baddoura F. K., Lakkis F. G. Acute rejection of vascularized heart allografts in the absence of IFNgamma. Transplantation. 1996 Dec 27;62(12):1908–1911. doi: 10.1097/00007890-199612270-00039. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., deBlois D., O'Brien E. R. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995 Sep;77(3):445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- Shi C., Russell M. E., Bianchi C., Newell J. B., Haber E. Murine model of accelerated transplant arteriosclerosis. Circ Res. 1994 Aug;75(2):199–207. doi: 10.1161/01.res.75.2.199. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay G., Ladel C. H., Blum C., Kaufmann S. H. IL-4 neutralization or TNF-alpha treatment ameliorate disease by an intracellular pathogen in IFN-gamma receptor-deficient mice. J Immunol. 1996 Dec 1;157(11):4746–4750. [PubMed] [Google Scholar]
- Tullius S. G., Tilney N. L. Both alloantigen-dependent and -independent factors influence chronic allograft rejection. Transplantation. 1995 Feb 15;59(3):313–318. [PubMed] [Google Scholar]
- Vermeire K., Heremans H., Vandeputte M., Huang S., Billiau A., Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997 Jun 1;158(11):5507–5513. [PubMed] [Google Scholar]
- Wang Z. E., Reiner S. L., Zheng S., Dalton D. K., Locksley R. M. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994 Apr 1;179(4):1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]




