Abstract
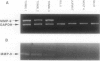
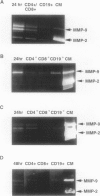
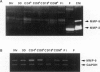
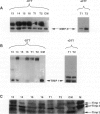
This study was conducted to assess the net proteolytic activity of human non-Hodgkin's lymphomas (NHLs). We have compared the extracellular matrix (ECM)-degradative abilities of human NHLs, reactive lymphoid hyperplasias, and established lymphoid cell lines using Matrigel invasion and elastin degradation assays. The inhibition studies allowed identification of the classes of proteinases involved in ECM degradation. Our results indicate that lymphocytes and other leukocytes derived from both human NHLs and reactive lymphoid hyperplasias are capable of Matrigel penetration, but only cells derived from the high-grade human NHLs degrade elastin in vitro. Established lymphoid cell lines (both malignant and Epstein-Barr virus immortalized) do not produce MMP-9, do not penetrate the Matrigel, and do not degrade elastin. Moreover, in human NHLs, elastolytic activity is blocked by metalloproteinase inhibitors, while inhibitors of the other classes of proteolytic enzymes have only minor effects. This study identifies metalloproteinases as the most important class of proteinases involved in ECM degradation by NHLs. The previous studies suggest that, within this class, MMP-9 represents the key enzyme that plays a role in the biological aggressiveness of human NHLs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batki S. L. Methadone maintenance. West J Med. 1989 Jun;150(6):717–717. [PMC free article] [PubMed] [Google Scholar]
- Belaaouaj A., Shipley J. M., Kobayashi D. K., Zimonjic D. B., Popescu N., Silverman G. A., Shapiro S. D. Human macrophage metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J Biol Chem. 1995 Jun 16;270(24):14568–14575. doi: 10.1074/jbc.270.24.14568. [DOI] [PubMed] [Google Scholar]
- Cottier H., Turk J., Sobin L. A proposal for a standardized system of reporting human lymph node morphology in relation to immunological function. Bull World Health Organ. 1972;47(3):375–417. [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn A., Hamann A. Mechanisms and regulation of lymphocyte migration. Immunol Today. 1989 Jan;10(1):23–28. doi: 10.1016/0167-5699(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Banda M. J., Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996 Jan 1;156(1):1–4. [PubMed] [Google Scholar]
- Hinek A., Rabinovitch M. The ductus arteriosus migratory smooth muscle cell phenotype processes tropoelastin to a 52-kDa product associated with impaired assembly of elastic laminae. J Biol Chem. 1993 Jan 15;268(2):1405–1413. [PubMed] [Google Scholar]
- Katsuda S., Okada Y., Okada Y., Imai K., Nakanishi I. Matrix metalloproteinase-9 (92-kd gelatinase/type IV collagenase equals gelatinase B) can degrade arterial elastin. Am J Pathol. 1994 Nov;145(5):1208–1218. [PMC free article] [PubMed] [Google Scholar]
- Khan K. M., Falcone D. J. Role of laminin in matrix induction of macrophage urokinase-type plasminogen activator and 92-kDa metalloproteinase expression. J Biol Chem. 1997 Mar 28;272(13):8270–8275. doi: 10.1074/jbc.272.13.8270. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Liotta L. A. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995 May 1;55(9):1856–1862. [PubMed] [Google Scholar]
- Kolkenbrock H., Hecker-Kia A., Orgel D., Kinawi A., Ulbrich N. Progelatinase B forms from human neutrophils. complex formation of monomer/lipocalin with TIMP-1. Biol Chem. 1996 Jul-Aug;377(7-8):529–533. doi: 10.1515/bchm3.1996.377.7-8.529. [DOI] [PubMed] [Google Scholar]
- Kossakowska A. E., Urbanski S. J., Edwards D. R. Tissue inhibitor of metalloproteinases-1 (TIMP-1) RNA is expressed at elevated levels in malignant non-Hodgkin's lymphomas. Blood. 1991 Jun 1;77(11):2475–2481. [PubMed] [Google Scholar]
- Kossakowska A. E., Urbanski S. J., Huchcroft S. A., Edwards D. R. Relationship between the clinical aggressiveness of large cell immunoblastic lymphomas and expression of 92 kDa gelatinase (type IV collagenase) and tissue inhibitor of metalloproteinases-1 (TIMP-1) RNAs. Oncol Res. 1992;4(6):233–240. [PubMed] [Google Scholar]
- Kossakowska A. E., Urbanski S. J., Watson A., Hayden L. J., Edwards D. R. Patterns of expression of metalloproteinases and their inhibitors in human malignant lymphomas. Oncol Res. 1993;5(1):19–28. [PubMed] [Google Scholar]
- Leake D. S., Hornebeck W., Bréchemier D., Robert L., Peters T. J. Properties and subcellular localization of elastase-like activities of arterial smooth muscle cells in culture. Biochim Biophys Acta. 1983 Nov 22;761(1):41–47. doi: 10.1016/0304-4165(83)90360-4. [DOI] [PubMed] [Google Scholar]
- Leco K. J., Khokha R., Pavloff N., Hawkes S. P., Edwards D. R. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem. 1994 Mar 25;269(12):9352–9360. [PubMed] [Google Scholar]
- Leppert D., Waubant E., Galardy R., Bunnett N. W., Hauser S. L. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995 May 1;154(9):4379–4389. [PubMed] [Google Scholar]
- Mainardi C. L., Pourmotabbed T. F., Hasty K. A. Inflammatory phagocytes and connective tissue degrading metalloproteinases. Am J Med Sci. 1991 Sep;302(3):171–175. doi: 10.1097/00000441-199109000-00010. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Rifkin D. B. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993 Jan;73(1):161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Pauli B. U., Knudson W. Tumor invasion: a consequence of destructive and compositional matrix alterations. Hum Pathol. 1988 Jun;19(6):628–639. doi: 10.1016/s0046-8177(88)80168-0. [DOI] [PubMed] [Google Scholar]
- Prosser I. W., Whitehouse L. A., Parks W. C., Stahle-Bäckdahl M., Hinek A., Park P. W., Mecham R. P. Polyclonal antibodies to tropoelastin and the specific detection and measurement of tropoelastin in vitro. Connect Tissue Res. 1991;25(3-4):265–279. doi: 10.3109/03008209109029162. [DOI] [PubMed] [Google Scholar]
- Ray J. M., Stetler-Stevenson W. G. The role of matrix metalloproteases and their inhibitors in tumour invasion, metastasis and angiogenesis. Eur Respir J. 1994 Nov;7(11):2062–2072. [PubMed] [Google Scholar]
- Sato H., Kida Y., Mai M., Endo Y., Sasaki T., Tanaka J., Seiki M. Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene. 1992 Jan;7(1):77–83. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Stauder R., Hamader S., Fasching B., Kemmler G., Thaler J., Huber H. Adhesion to high endothelial venules: a model for dissemination mechanisms in non-Hodgkin's lymphoma. Blood. 1993 Jul 1;82(1):262–267. [PubMed] [Google Scholar]
- Stetler-Stevenson M., Mansoor A., Lim M., Fukushima P., Kehrl J., Marti G., Ptaszynski K., Wang J., Stetler-Stevenson W. G. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood. 1997 Mar 1;89(5):1708–1715. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996 May;148(5):1345–1350. [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Hewitt R., Corcoran M. Matrix metalloproteinases and tumor invasion: from correlation and causality to the clinic. Semin Cancer Biol. 1996 Jun;7(3):147–154. doi: 10.1006/scbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. TIMP-2: identification and characterization of a new member of the metalloproteinase inhibitor family. Matrix Suppl. 1992;1:299–306. [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Sudbeck B. D., Eisen A. Z., Welgus H. G., Parks W. C. Expression of 92-kDa type IV collagenase mRNA by eosinophils associated with basal cell carcinoma. J Invest Dermatol. 1992 Oct;99(4):497–503. doi: 10.1111/1523-1747.ep12616171. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Nakanishi I., Yamashita K., Hayakawa T., Okada Y. Matrix metalloproteinase-9 (92 kDa gelatinase/type IV collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci. 1993 Apr;104(Pt 4):991–999. doi: 10.1242/jcs.104.4.991. [DOI] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Wong H., Anderson W. D., Cheng T., Riabowol K. T. Monitoring mRNA expression by polymerase chain reaction: the "primer-dropping" method. Anal Biochem. 1994 Dec;223(2):251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- Zhou H., Bernhard E. J., Fox F. E., Billings P. C. Induction of metalloproteinase activity in human T-lymphocytes. Biochim Biophys Acta. 1993 Jun 6;1177(2):174–178. doi: 10.1016/0167-4889(93)90037-p. [DOI] [PubMed] [Google Scholar]