Abstract
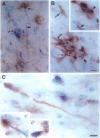
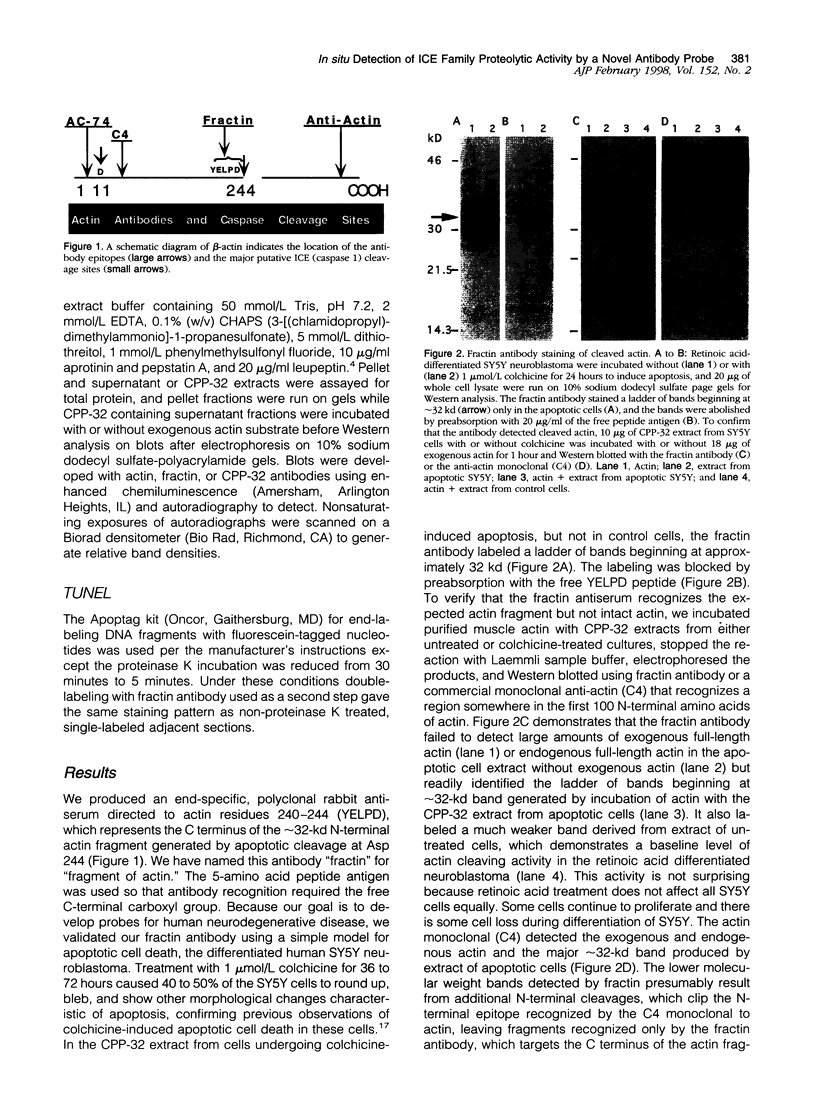
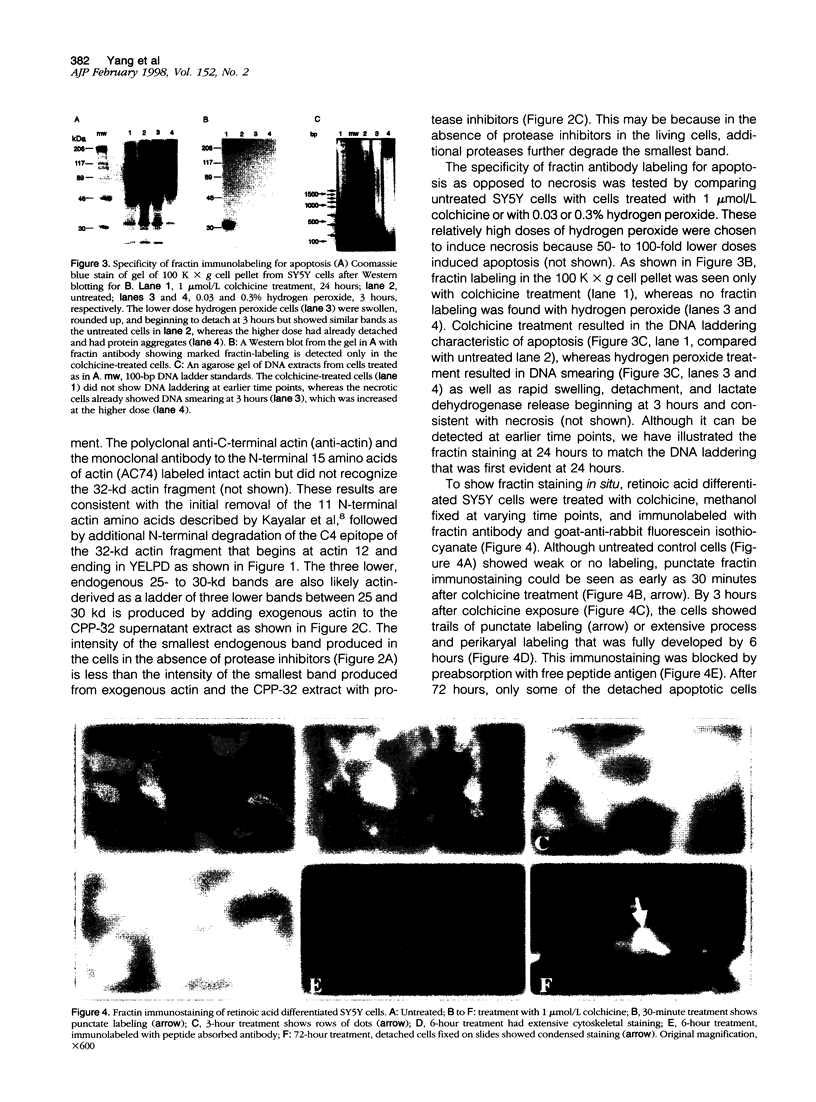
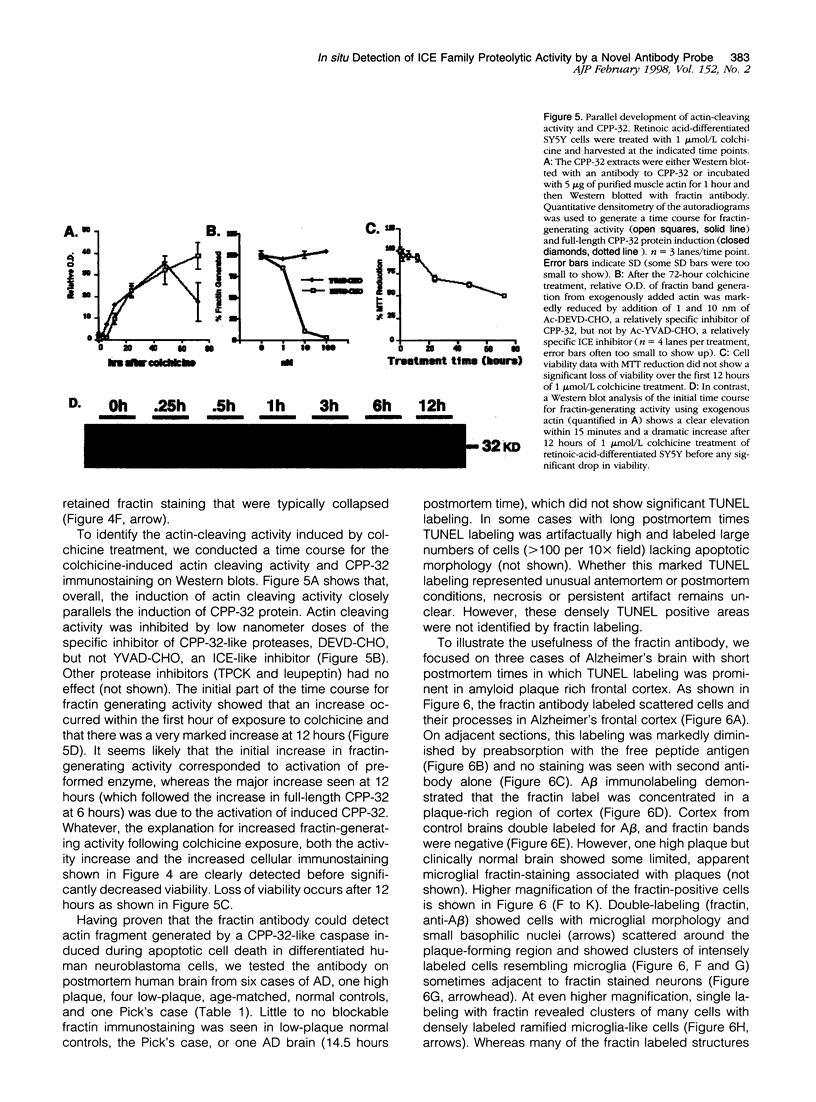
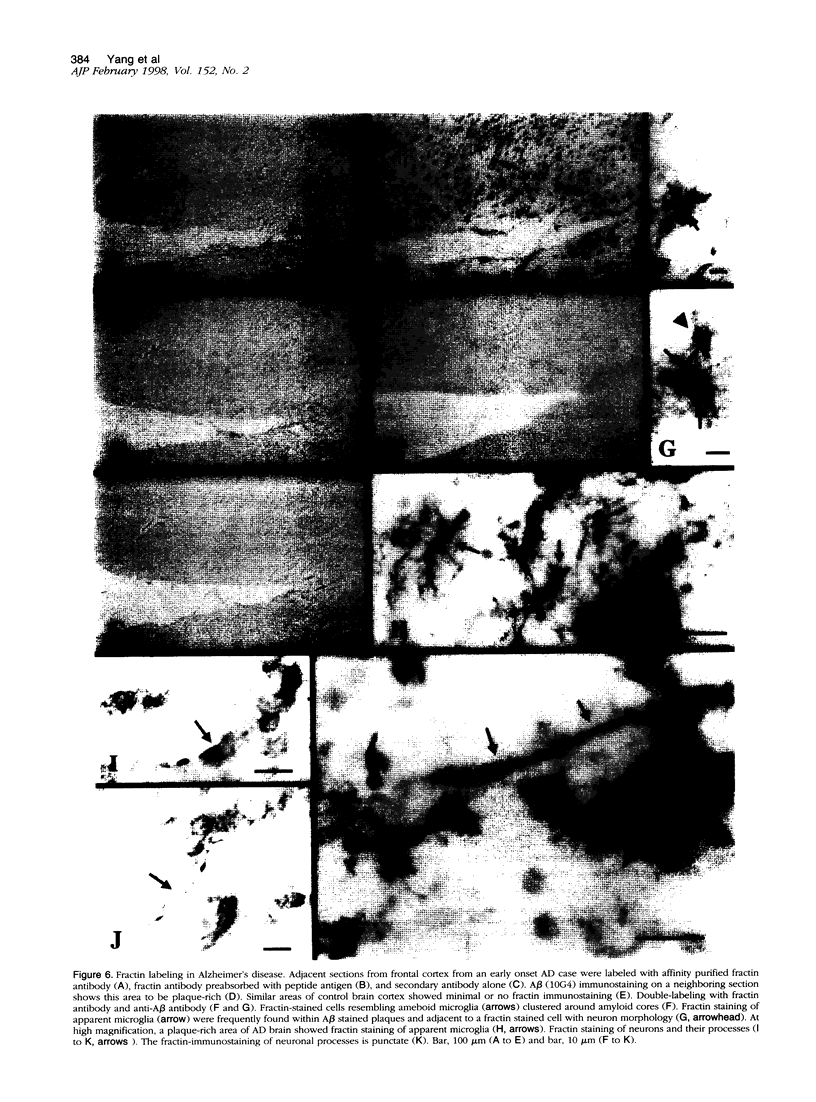
During apoptosis, activation of a family of cysteine proteases related to interleukin-1beta-converting enzyme (ICE)-related proteases or "caspases" results in endoproteolytic cleavage of multiple substrates at specific aspartate residues. We have sought to develop new antibody probes for the neoepitopes in protein fragments produced by ICE-related proteolytic cleavage as specific markers of events tightly linked to apoptotic mechanisms. Here, we demonstrate that an antibody probe specific for the C terminus of a 32-kd actin fragment produced by ICE-like activity specifically labels apoptotic but not necrotic, differentiated human neuroblastoma cells in culture. Unlike probes for nonspecific DNA strand breaks confined to the nucleus or cell body, this method allows the detection of cytoskeletal fragments in cell processes as well as the perikaryon long before DNA fragmentation and cell death and therefore serves as a novel marker of apoptosis-related events in distal parts of cells such as axons and dendrites. To illustrate this new tool, we show that the antibody detects the processes and cell bodies of degenerating neurons and plaque-associated microglia in Alzheimer's disease. In situ detection of caspase-cleaved actin provides a new means to evaluate the role of caspase activation in pathological and physiological processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat R. V., DiRocco R., Marcy V. R., Flood D. G., Zhu Y., Dobrzanski P., Siman R., Scott R., Contreras P. C., Miller M. Increased expression of IL-1beta converting enzyme in hippocampus after ischemia: selective localization in microglia. J Neurosci. 1996 Jul 1;16(13):4146–4154. doi: 10.1523/JNEUROSCI.16-13-04146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charriaut-Marlangue C., Ben-Ari Y. A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport. 1995 Dec 29;7(1):61–64. [PubMed] [Google Scholar]
- Columbano A. Cell death: current difficulties in discriminating apoptosis from necrosis in the context of pathological processes in vivo. J Cell Biochem. 1995 Jun;58(2):181–190. doi: 10.1002/jcb.240580207. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Anderson A. J. A potential role for apoptosis in neurodegeneration and Alzheimer's disease. Mol Neurobiol. 1995 Feb;10(1):19–45. doi: 10.1007/BF02740836. [DOI] [PubMed] [Google Scholar]
- Cotter T. G., Lennon S. V., Glynn J. M., Green D. R. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res. 1992 Feb 15;52(4):997–1005. [PubMed] [Google Scholar]
- Enari M., Talanian R. V., Wong W. W., Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996 Apr 25;380(6576):723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Endresen P. C., Fandrem J., Eide T. J., Aarbakke J. Morphological modifications of apoptosis in HL-60 cells: effects of homocysteine and cytochalasins on apoptosis initiated by 3-deazaadenosine. Virchows Arch. 1995;426(3):257–266. doi: 10.1007/BF00191363. [DOI] [PubMed] [Google Scholar]
- Estus S., Zaks W. J., Freeman R. S., Gruda M., Bravo R., Johnson E. M., Jr Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994 Dec;127(6 Pt 1):1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J., Banati R. B. Microglial turnover in the injured CNS: activated microglia undergo delayed DNA fragmentation following peripheral nerve injury. J Neuropathol Exp Neurol. 1995 Sep;54(5):680–688. doi: 10.1097/00005072-199509000-00010. [DOI] [PubMed] [Google Scholar]
- Iyer R., Hamilton R. F., Li L., Holian A. Silica-induced apoptosis mediated via scavenger receptor in human alveolar macrophages. Toxicol Appl Pharmacol. 1996 Nov;141(1):84–92. doi: 10.1006/taap.1996.0263. [DOI] [PubMed] [Google Scholar]
- Kayalar C., Ord T., Testa M. P., Zhong L. T., Bredesen D. E. Cleavage of actin by interleukin 1 beta-converting enzyme to reverse DNase I inhibition. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):2234–2238. doi: 10.1073/pnas.93.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotzer A. R., Pike C. J., Cotman C. W. beta-Amyloid peptides induce degeneration of cultured rat microglia. Brain Res. 1993 Oct 8;624(1-2):121–125. doi: 10.1016/0006-8993(93)90068-x. [DOI] [PubMed] [Google Scholar]
- Kuida K., Zheng T. S., Na S., Kuan C., Yang D., Karasuyama H., Rakic P., Flavell R. A. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996 Nov 28;384(6607):368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Lasorella A., Iavarone A., Israel M. A. Differentiation of neuroblastoma enhances Bcl-2 expression and induces alterations of apoptosis and drug resistance. Cancer Res. 1995 Oct 15;55(20):4711–4716. [PubMed] [Google Scholar]
- Lassmann H., Bancher C., Breitschopf H., Wegiel J., Bobinski M., Jellinger K., Wisniewski H. M. Cell death in Alzheimer's disease evaluated by DNA fragmentation in situ. Acta Neuropathol. 1995;89(1):35–41. doi: 10.1007/BF00294257. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y. A., Takahashi A., Moir R. D., Goldman R. D., Poirier G. G., Kaufmann S. H., Earnshaw W. C. Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9042–9046. doi: 10.1073/pnas.92.20.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J. Discovery and characterization of two novel human cancer-related proteins using two-dimensional gel electrophoresis. Electrophoresis. 1994 Mar-Apr;15(3-4):345–357. doi: 10.1002/elps.1150150154. [DOI] [PubMed] [Google Scholar]
- Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995 Feb 10;80(3):401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Deuschle U., Brockhaus M., Reinhardt D., Nelboeck P., Mous J., Grünberg J., Haass C., Jacobsen H. Presenilins are processed by caspase-type proteases. J Biol Chem. 1997 Aug 15;272(33):20655–20659. doi: 10.1074/jbc.272.33.20655. [DOI] [PubMed] [Google Scholar]
- Mak K., Yang F., Vinters H. V., Frautschy S. A., Cole G. M. Polyclonals to beta-amyloid(1-42) identify most plaque and vascular deposits in Alzheimer cortex, but not striatum. Brain Res. 1994 Dec 19;667(1):138–142. doi: 10.1016/0006-8993(94)91725-6. [DOI] [PubMed] [Google Scholar]
- Mashima T., Naito M., Fujita N., Noguchi K., Tsuruo T. Identification of actin as a substrate of ICE and an ICE-like protease and involvement of an ICE-like protease but not ICE in VP-16-induced U937 apoptosis. Biochem Biophys Res Commun. 1995 Dec 26;217(3):1185–1192. doi: 10.1006/bbrc.1995.2894. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995 Sep;21(2):195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Melino G., Thiele C. J., Knight R. A., Piacentini M. Retinoids and the control of growth/death decisions in human neuroblastoma cell lines. J Neurooncol. 1997 Jan;31(1-2):65–83. doi: 10.1023/a:1005733430435. [DOI] [PubMed] [Google Scholar]
- Miller D. K. The role of the Caspase family of cysteine proteases in apoptosis. Semin Immunol. 1997 Feb;9(1):35–49. doi: 10.1006/smim.1996.0058. [DOI] [PubMed] [Google Scholar]
- Miossec C., Dutilleul V., Fassy F., Diu-Hercend A. Evidence for CPP32 activation in the absence of apoptosis during T lymphocyte stimulation. J Biol Chem. 1997 May 23;272(21):13459–13462. doi: 10.1074/jbc.272.21.13459. [DOI] [PubMed] [Google Scholar]
- Mundle S. D., Raza A. The two in situ techniques do not differentiate between apoptosis and necrosis but rather reveal distinct patterns of DNA fragmentation in apoptosis. Lab Invest. 1995 May;72(5):611–613. [PubMed] [Google Scholar]
- Nakagawa-Yagi Y. Induction of apoptotic cell death in differentiating neuroblastoma SH-SY5Y cells by colchicine. Biochem Biophys Res Commun. 1994 Mar 15;199(2):807–817. doi: 10.1006/bbrc.1994.1301. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Golstein P., Henkart P. A. The target cell nucleus is not required for cell-mediated granzyme- or Fas-based cytotoxicity. J Exp Med. 1995 May 1;181(5):1905–1909. doi: 10.1084/jem.181.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995 Jul 6;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Paresce D. M., Ghosh R. N., Maxfield F. R. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996 Sep;17(3):553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C., Price D. L., Martin L. J. Excitotoxic neuronal death in the immature brain is an apoptosis-necrosis morphological continuum. J Comp Neurol. 1997 Feb 3;378(1):70–87. [PubMed] [Google Scholar]
- Schlegel J., Peters I., Orrenius S., Miller D. K., Thornberry N. A., Yamin T. T., Nicholson D. W. CPP32/apopain is a key interleukin 1 beta converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996 Jan 26;271(4):1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Walczak H., Dröge W., Krammer P. H. Cell nucleus and DNA fragmentation are not required for apoptosis. J Cell Biol. 1994 Oct;127(1):15–20. doi: 10.1083/jcb.127.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale G., Nichols N. R., Brady D. R., Finch C. E., Horton W. E., Jr Evidence for apoptotic cell death in Alzheimer's disease. Exp Neurol. 1995 Jun;133(2):225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Song Q., Wei T., Lees-Miller S., Alnemri E., Watters D., Lavin M. F. Resistance of actin to cleavage during apoptosis. Proc Natl Acad Sci U S A. 1997 Jan 7;94(1):157–162. doi: 10.1073/pnas.94.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Su J. H., Anderson A. J., Cummings B. J., Cotman C. W. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport. 1994 Dec 20;5(18):2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- Vanderklish P., Saido T. C., Gall C., Arai A., Lynch G. Proteolysis of spectrin by calpain accompanies theta-burst stimulation in cultured hippocampal slices. Brain Res Mol Brain Res. 1995 Aug;32(1):25–35. doi: 10.1016/0169-328x(95)00057-y. [DOI] [PubMed] [Google Scholar]
- Vito P., Wolozin B., Ganjei J. K., Iwasaki K., Lacanà E., D'Adamio L. Requirement of the familial Alzheimer's disease gene PS2 for apoptosis. Opposing effect of ALG-3. J Biol Chem. 1996 Dec 6;271(49):31025–31028. doi: 10.1074/jbc.271.49.31025. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Iwasaki K., Vito P., Ganjei J. K., Lacanà E., Sunderland T., Zhao B., Kusiak J. W., Wasco W., D'Adamio L. Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science. 1996 Dec 6;274(5293):1710–1713. doi: 10.1126/science.274.5293.1710. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yang F., Mak K., Vinters H. V., Frautschy S. A., Cole G. M. Monoclonal antibody to the C-terminus of beta-amyloid. Neuroreport. 1994 Oct 27;5(16):2117–2120. doi: 10.1097/00001756-199410270-00032. [DOI] [PubMed] [Google Scholar]