Abstract
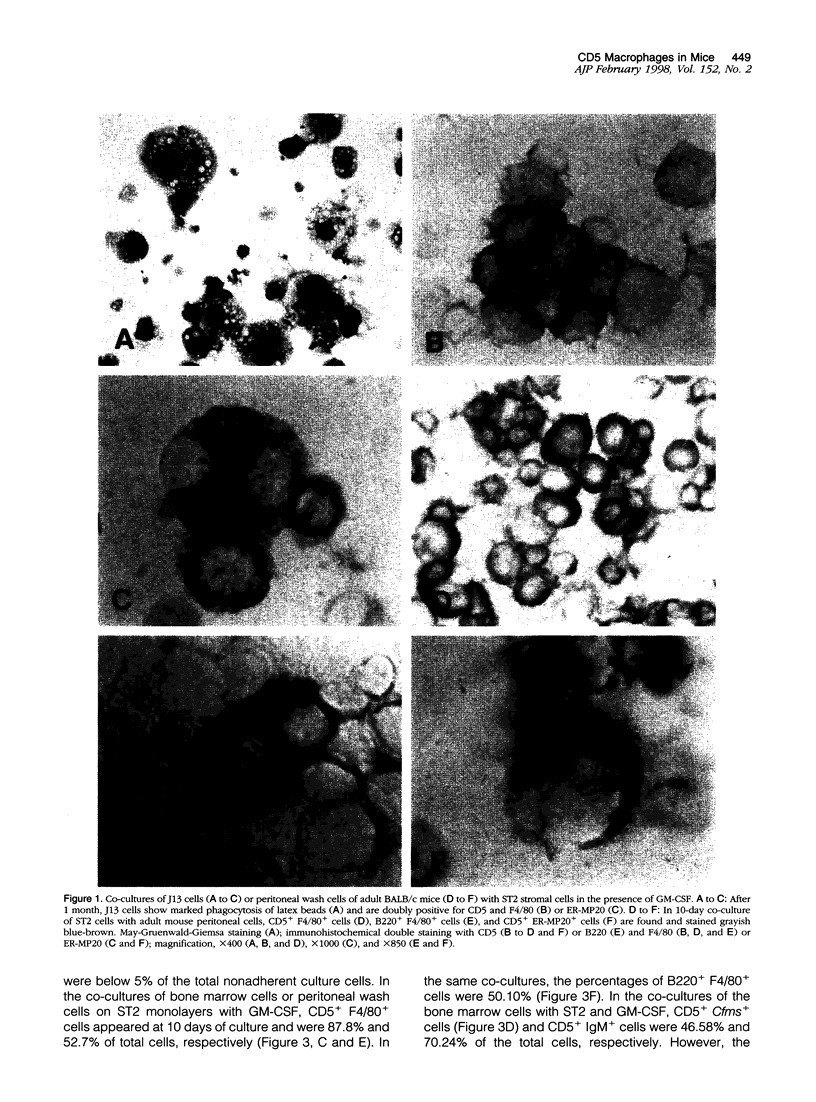
In co-cultures of either the murine pre-B cell line J13, fetal liver cells, or adult peritoneal or bone marrow cells with ST2 mouse bone marrow stromal cells in the presence of granulocyte/macrophage colony-stimulating factor (GM-CSF), the development of CD5+ macrophages was demonstrated by immunohistochemical staining and flow cytometry. Although CD5+ macrophages were not present in the peritoneal cavities of normal mice, approximately 30% of the peritoneal macrophages in viable motheaten (mev/mev) mice, deficient in SHP-1 protein tyrosine phosphatase, expressed cell surface CD5 and B220, markers for B cells. In the mev/mev mice, GM-CSF level in peritoneal fluid was increased significantly. At 5 days after daily intravenous injection with GM-CSF, many CD5+ macrophages appeared in the peritoneal cavity and in omental milky spots of normal mice but fewer in osteopetrosis (op) mutant mice, deficient in macrophage (M)-CSF. These results indicate that GM-CSF, in combination with M-CSF, induces the development and differentiation of CD5+ macrophages in the peritoneal cavity, particularly in the omental milky spots of mice. In the peritoneal cavity of GM-CSF-treated mice, the percentages of hematopoietic progenitor cells doubly positive for CD5 and CD34 or c-kit and of macrophage precursor cells doubly positive for CD5 and ER-MP58 or ER-MP20 were increased significantly during the development of CD5+ macrophages and CD5 B cells, suggesting that CD5+ macrophages and B cells may share a bipotential progenitor in vivo.
Full text
PDF



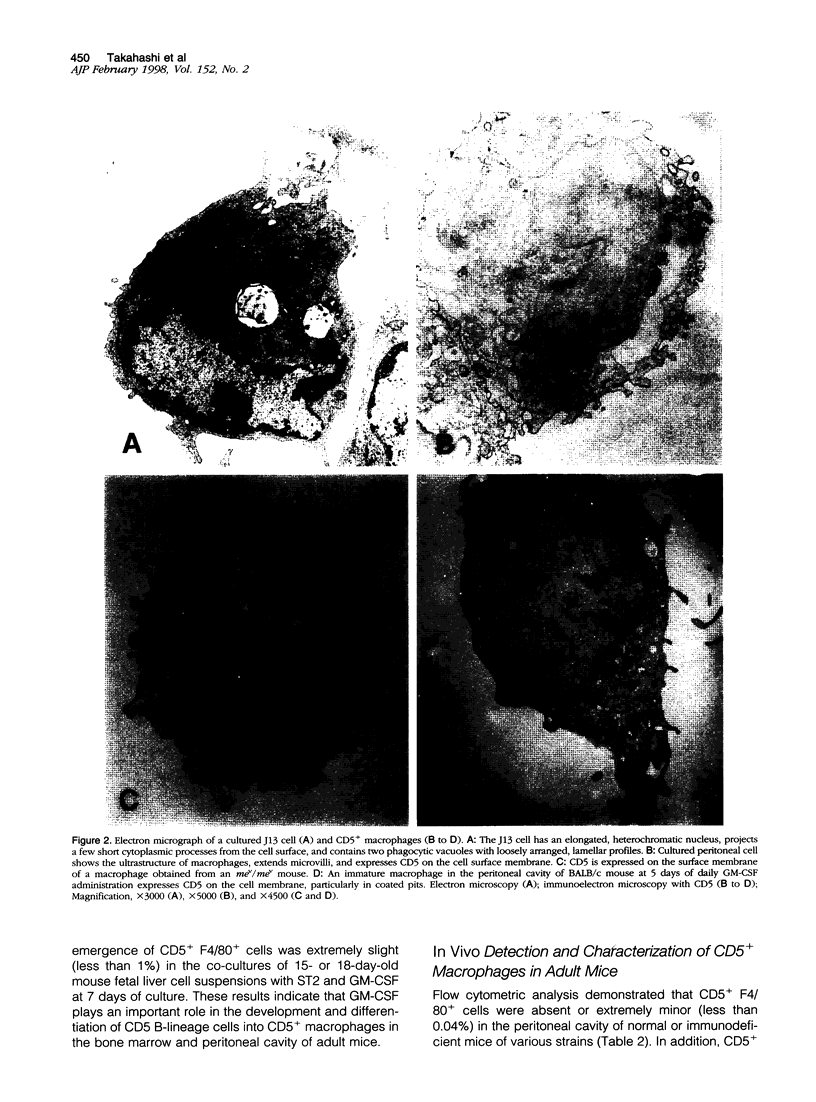
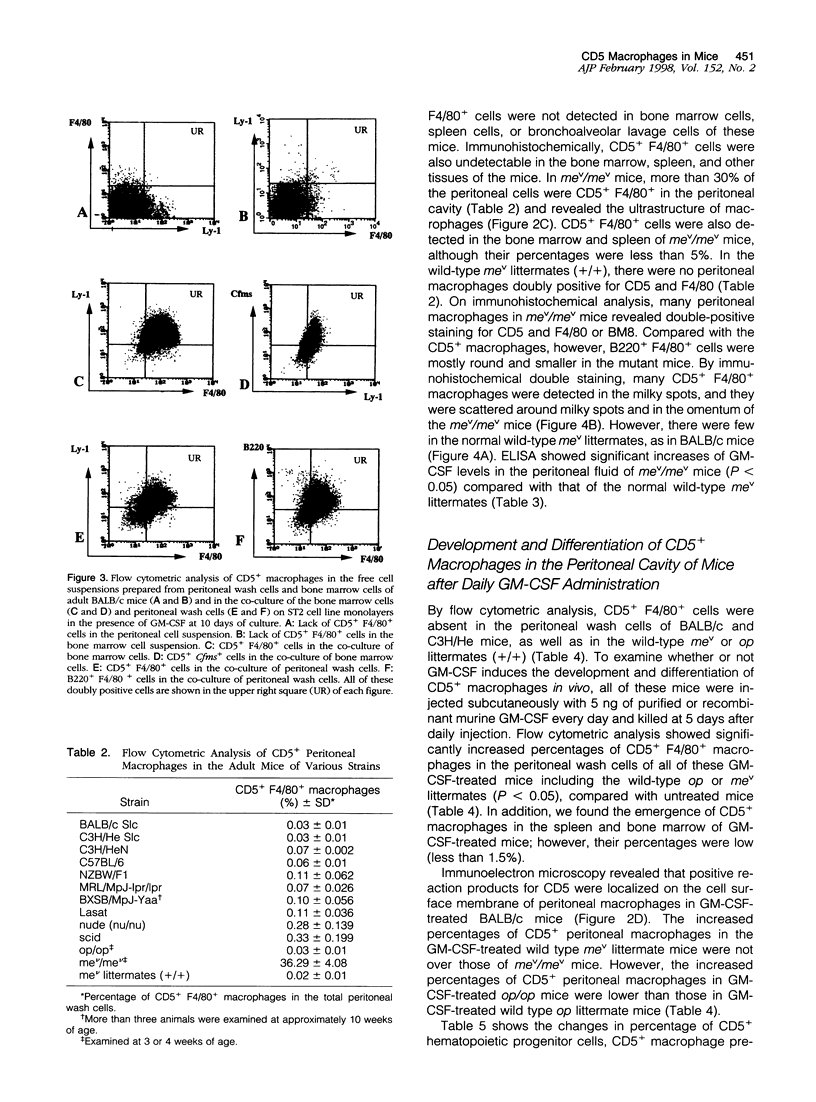
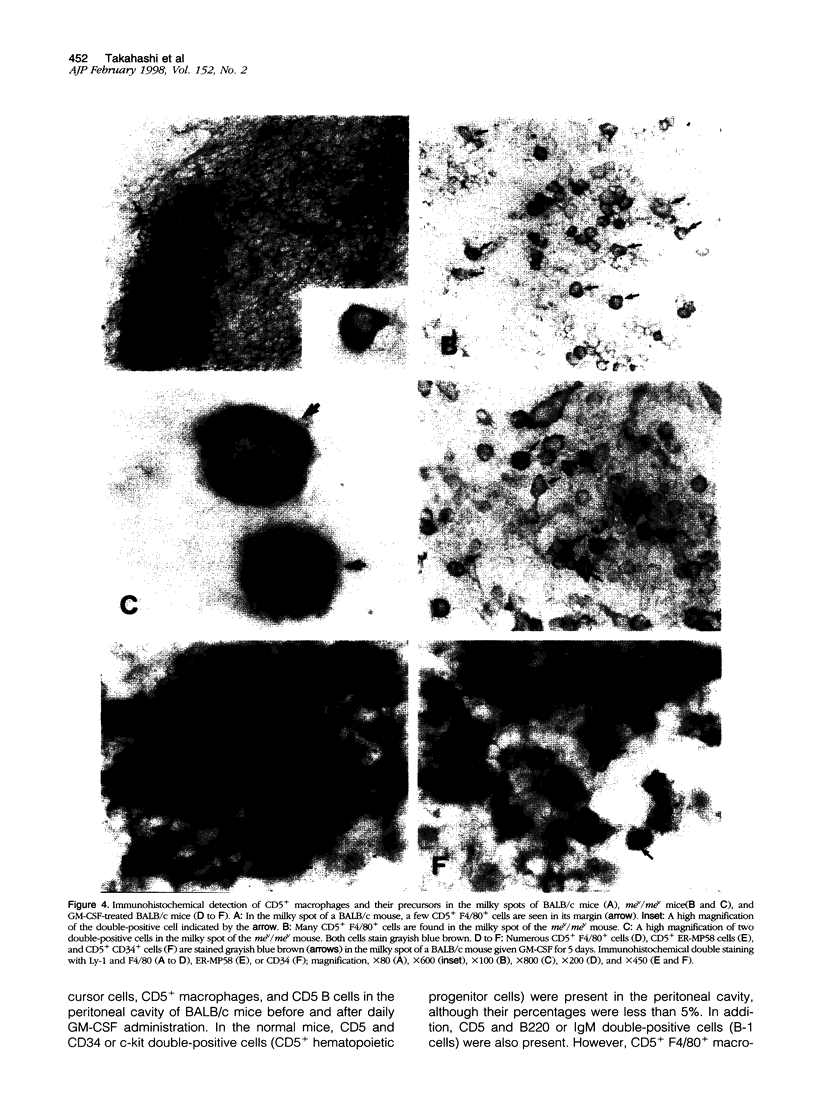
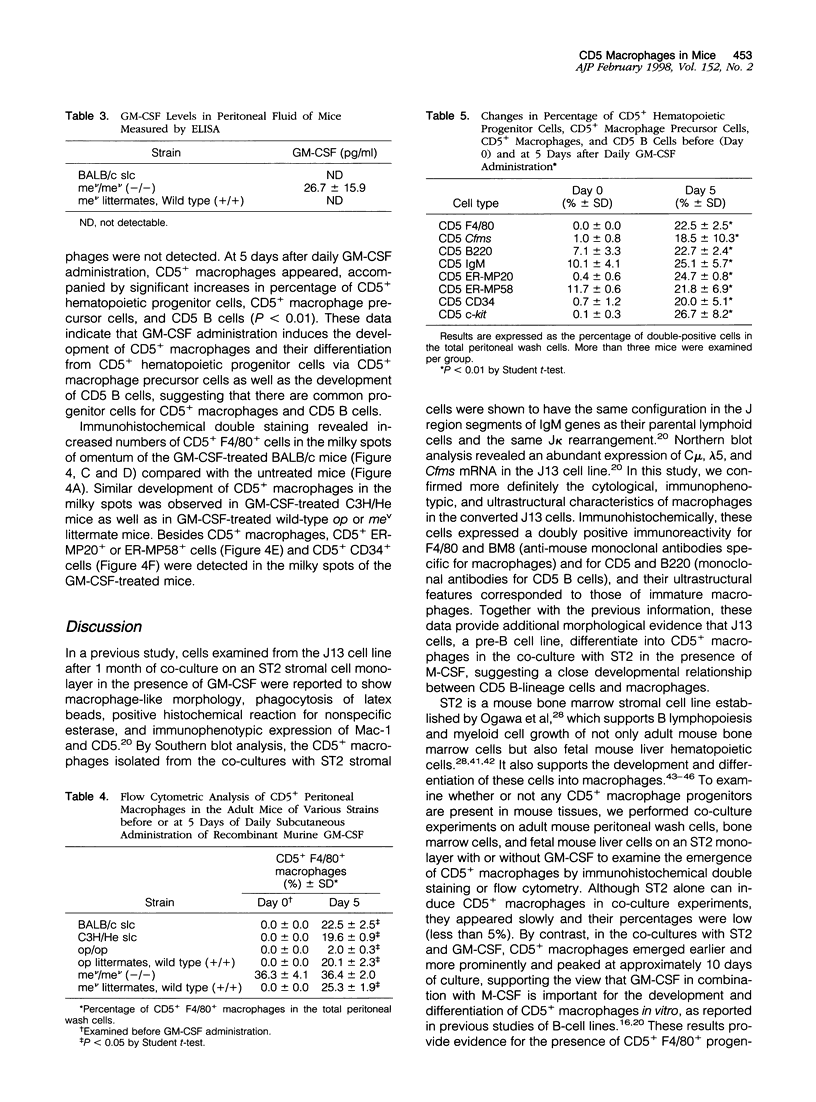



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bagley C. J., Woodcock J. M., Hercus T. R., Shannon M. F., Lopez A. F. Interaction of GM-CSF and IL-3 with the common beta-chain of their receptors. J Leukoc Biol. 1995 May;57(5):739–746. doi: 10.1002/jlb.57.5.739. [DOI] [PubMed] [Google Scholar]
- Bauer S. R., Holmes K. L., Morse H. C., 3rd, Potter M. Clonal relationship of the lymphoblastic cell line P388 to the macrophage cell line P388D1 as evidenced by immunoglobulin gene rearrangements and expression of cell surface antigens. J Immunol. 1986 Jun 15;136(12):4695–4699. [PubMed] [Google Scholar]
- Beelen R. H., Fluitsma D. M., Hoefsmit E. C. Peroxidatic activity of mononuclear phagocytes developing in omentum milky spots. J Reticuloendothel Soc. 1980 Dec;28(6):601–609. [PubMed] [Google Scholar]
- Beelen R. H., Fluitsma D. M., Hoefsmit E. C. The cellular composition of omentum milky spots and the ultrastructure of milky spot macrophages and reticulum cells. J Reticuloendothel Soc. 1980 Dec;28(6):585–599. [PubMed] [Google Scholar]
- Boak J. L., Christie G. H., Ford W. L., Howard J. G. Pathways in the development of liver macrophages: alternative precursors contained in populations of lymphocytes and bone-marrow cells. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):307–327. doi: 10.1098/rspb.1968.0013. [DOI] [PubMed] [Google Scholar]
- Borrello M. A., Phipps R. P. The B/macrophage cell: an elusive link between CD5+ B lymphocytes and macrophages. Immunol Today. 1996 Oct;17(10):471–475. doi: 10.1016/0167-5699(96)20031-b. [DOI] [PubMed] [Google Scholar]
- Borzillo G. V., Ashmun R. A., Sherr C. J. Macrophage lineage switching of murine early pre-B lymphoid cells expressing transduced fms genes. Mol Cell Biol. 1990 Jun;10(6):2703–2714. doi: 10.1128/mcb.10.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A. W., Schrader J. W. Derivation of macrophage-like lines from the pre-B lymphoma ABLS 8.1 using 5-azacytidine. Nature. 1982 Jun 24;297(5868):691–693. doi: 10.1038/297691a0. [DOI] [PubMed] [Google Scholar]
- Bretz J. D., Chen S. C., Schwartz R. C. Antigen presentation after macrophage lineage switch of CD5 pre-B cells. Ann N Y Acad Sci. 1992 May 4;651:155–156. doi: 10.1111/j.1749-6632.1992.tb24605.x. [DOI] [PubMed] [Google Scholar]
- Carr I. The fine structure of the cells of the mouse peritoneum. Z Zellforsch Mikrosk Anat. 1967;80(4):534–555. doi: 10.1007/BF00330721. [DOI] [PubMed] [Google Scholar]
- Chen H. E., Chang S., Trub T., Neel B. G. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1996 Jul;16(7):3685–3697. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranshaw M. L., Leak L. V. Milky spots of the omentum: a source of peritoneal cells in the normal and stimulated animal. Arch Histol Cytol. 1990;53 (Suppl):165–177. doi: 10.1679/aohc.53.suppl_165. [DOI] [PubMed] [Google Scholar]
- Cumano A., Paige C. J., Iscove N. N., Brady G. Bipotential precursors of B cells and macrophages in murine fetal liver. Nature. 1992 Apr 16;356(6370):612–615. doi: 10.1038/356612a0. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Pierce J. H., Holmes K. L. Evidence for a developmental relationship between CD5+ B-lineage cells and macrophages. Ann N Y Acad Sci. 1992 May 4;651:112–129. doi: 10.1111/j.1749-6632.1992.tb24601.x. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Pierce J. H., Rudikoff S., Morse H. C., 3rd Relationships between B cell and myeloid differentiation. Studies with a B lymphocyte progenitor line, HAFTL-1. J Exp Med. 1988 Jul 1;168(1):389–407. doi: 10.1084/jem.168.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. G., Yang Y. C., Clark S. C., Long M. W. Human recombinant granulocyte-macrophage colony stimulating factor and interleukin 3 have overlapping but distinct hematopoietic activities. J Clin Invest. 1988 Oct;82(4):1282–1287. doi: 10.1172/JCI113727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUGH J., ELVES M. W., ISRAUELS M. C. THE FORMATION OF MACROPHAGES FROM LYMPHOCYTES IN VITRO. Exp Cell Res. 1965 Jun;38:476–482. doi: 10.1016/0014-4827(65)90371-x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Kantor A. B., Herzenberg L. A. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci. 1992 May 4;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- Higashi K., Naito M., Takeya M., Ando M., Araki S., Takahashi K. Ontogenetic development, differentiation, and phenotypic expression of macrophages in fetal rat lungs. J Leukoc Biol. 1992 May;51(5):444–454. doi: 10.1002/jlb.51.5.444. [DOI] [PubMed] [Google Scholar]
- Holmes K. L., Pierce J. H., Davidson W. F., Morse H. C., 3rd Murine hematopoietic cells with pre-B or pre-B/myeloid characteristics are generated by in vitro transformation with retroviruses containing fes, ras, abl, and src oncogenes. J Exp Med. 1986 Aug 1;164(2):443–457. doi: 10.1084/jem.164.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub M., Hajdu I., Trebichavský I., Jarosková L. Formation of lymphoid cells from local precursors in irradiated mouse omenta. Eur J Immunol. 1971 Dec;1(6):465–470. doi: 10.1002/eji.1830010612. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H., Yang W., Berrada K., Tabrizi M., Shultz L., Yi T. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative responses to GM-CSF: detection of potential HCP substrates in GM-CSF signal transduction. Exp Hematol. 1997 Jul;25(7):592–600. [PubMed] [Google Scholar]
- Katoh S., Tominaga A., Migita M., Kudo A., Takatsu K. Conversion of normal Ly-1-positive B-lineage cells into Ly-1-positive macrophages in long-term bone marrow cultures. Dev Immunol. 1990;1(2):113–125. doi: 10.1155/1990/28760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinken S. P., Alexander W. S., Adams J. M. Hemopoietic lineage switch: v-raf oncogene converts Emu-myc transgenic B cells into macrophages. Cell. 1988 Jun 17;53(6):857–867. doi: 10.1016/s0092-8674(88)90309-1. [DOI] [PubMed] [Google Scholar]
- Kubai L., Auerbach R. A new source of embryonic lymphocytes in the mouse. Nature. 1983 Jan 13;301(5896):154–156. doi: 10.1038/301154a0. [DOI] [PubMed] [Google Scholar]
- Leenen P. J., Melis M., Slieker W. A., Van Ewijk W. Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. Eur J Immunol. 1990 Jan;20(1):27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- Leenen P. J., de Bruijn M. F., Voerman J. S., Campbell P. A., van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994 Sep 14;174(1-2):5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Malorny U., Michels E., Sorg C. A monoclonal antibody against an antigen present on mouse macrophages and absent from monocytes. Cell Tissue Res. 1986;243(2):421–428. doi: 10.1007/BF00251059. [DOI] [PubMed] [Google Scholar]
- Morioka Y., Naito M., Sato T., Takahashi K. Immunophenotypic and ultrastructural heterogeneity of macrophage differentiation in bone marrow and fetal hematopoiesis of mouse in vitro and in vivo. J Leukoc Biol. 1994 May;55(5):642–651. doi: 10.1002/jlb.55.5.642. [DOI] [PubMed] [Google Scholar]
- Naito M., Hayashi S., Yoshida H., Nishikawa S., Shultz L. D., Takahashi K. Abnormal differentiation of tissue macrophage populations in 'osteopetrosis' (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1991 Sep;139(3):657–667. [PMC free article] [PubMed] [Google Scholar]
- Naito M., Takahashi K., Nishikawa S. Development, differentiation, and maturation of macrophages in the fetal mouse liver. J Leukoc Biol. 1990 Jul;48(1):27–37. doi: 10.1002/jlb.48.1.27. [DOI] [PubMed] [Google Scholar]
- Naito M., Yamamura F., Nishikawa S., Takahashi K. Development, differentiation, and maturation of fetal mouse yolk sac macrophages in cultures. J Leukoc Biol. 1989 Jul;46(1):1–10. doi: 10.1002/jlb.46.1.1. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Ogawa M., Nishikawa S., Kunisada T., Kodama H. B lymphopoiesis on stromal cell clone: stromal cell clones acting on different stages of B cell differentiation. Eur J Immunol. 1988 Nov;18(11):1767–1771. doi: 10.1002/eji.1830181117. [DOI] [PubMed] [Google Scholar]
- Nishinakamura R., Nakayama N., Hirabayashi Y., Inoue T., Aud D., McNeil T., Azuma S., Yoshida S., Toyoda Y., Arai K. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity. 1995 Mar;2(3):211–222. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Okada S., Nakauchi H., Nagayoshi K., Nishikawa S., Miura Y., Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992 Dec 15;80(12):3044–3050. [PubMed] [Google Scholar]
- Okada S., Nakauchi H., Nagayoshi K., Nishikawa S., Nishikawa S., Miura Y., Suda T. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood. 1991 Oct 1;78(7):1706–1712. [PubMed] [Google Scholar]
- Osawa M., Hanada K., Hamada H., Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996 Jul 12;273(5272):242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Principato M., Cleveland J. L., Rapp U. R., Holmes K. L., Pierce J. H., Morse H. C., 3rd, Klinken S. P. Transformation of murine bone marrow cells with combined v-raf-v-myc oncogenes yields clonally related mature B cells and macrophages. Mol Cell Biol. 1990 Jul;10(7):3562–3568. doi: 10.1128/mcb.10.7.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REBUCK J. W., CROWLEY J. H. A method of studying leukocytic functions in vivo. Ann N Y Acad Sci. 1955 Mar 24;59(5):757–805. doi: 10.1111/j.1749-6632.1955.tb45983.x. [DOI] [PubMed] [Google Scholar]
- Ratajczak M. Z., Jaskulski D., Pojda Z., Wiktor-Jedrzejczak W. Omental lymphoid organ as a source of macrophage colony stimulating activity in peritoneal cavity. Clin Exp Immunol. 1987 Jul;69(1):198–203. [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Seremetis S. V., Pelicci P. G., Tabilio A., Ubriaco A., Grignani F., Cuttner J., Winchester R. J., Knowles D. M., 2nd, Dalla-Favera R. High frequency of clonal immunoglobulin or T cell receptor gene rearrangements in acute myelogenous leukemia expressing terminal deoxyribonucleotidyltransferase. J Exp Med. 1987 Jun 1;165(6):1703–1712. doi: 10.1084/jem.165.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Rajan T. V., Yi T., Ihle J. N., Matthews R. J., Thomas M. L., Beier D. R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993 Jul 2;73(7):1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Solvason N., Chen X., Shu F., Kearney J. F. The fetal omentum in mice and humans. A site enriched for precursors of CD5 B cells early in development. Ann N Y Acad Sci. 1992 May 4;651:10–20. doi: 10.1111/j.1749-6632.1992.tb24589.x. [DOI] [PubMed] [Google Scholar]
- Sonoda Y., Yang Y. C., Wong G. G., Clark S. C., Ogawa M. Analysis in serum-free culture of the targets of recombinant human hemopoietic growth factors: interleukin 3 and granulocyte/macrophage-colony-stimulating factor are specific for early developmental stages. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4360–4364. doi: 10.1073/pnas.85.12.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall A. M., Adams S., Herzenberg L. A., Kantor A. B. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann N Y Acad Sci. 1992 May 4;651:33–43. doi: 10.1111/j.1749-6632.1992.tb24591.x. [DOI] [PubMed] [Google Scholar]
- Sudo T., Ito M., Ogawa Y., Iizuka M., Kodama H., Kunisada T., Hayashi S., Ogawa M., Sakai K., Nishikawa S. Interleukin 7 production and function in stromal cell-dependent B cell development. J Exp Med. 1989 Jul 1;170(1):333–338. doi: 10.1084/jem.170.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Naito M., Umeda S., Shultz L. D. The role of macrophage colony-stimulating factor in hepatic glucan-induced granuloma formation in the osteopetrosis mutant mouse defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1994 Jun;144(6):1381–1392. [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tominaga A. Interleukin 5 and its receptor. Prog Growth Factor Res. 1991;3(2):87–102. doi: 10.1016/s0955-2235(05)80001-8. [DOI] [PubMed] [Google Scholar]
- Takemori N., Hirai K., Onodera R., Saito N., Namiki M. Light and electron microscopic study of omental milky spots in New Zealand black mice, with special reference to the extramedullary hematopoiesis. Anat Embryol (Berl) 1994 Mar;189(3):215–226. doi: 10.1007/BF00239009. [DOI] [PubMed] [Google Scholar]
- Tominaga A., Mita S., Kikuchi Y., Hitoshi Y., Takatsu K., Nishikawa S., Ogawa M. Establishment of IL-5-dependent early B cell lines by long-term bone marrow cultures. Growth Factors. 1989;1(2):135–146. doi: 10.3109/08977198909029123. [DOI] [PubMed] [Google Scholar]
- Umeda S., Takahashi K., Shultz L. D., Naito M., Takagi K. Effects of macrophage colony-stimulating factor on macrophages and their related cell populations in the osteopetrosis mouse defective in production of functional macrophage colony-stimulating factor protein. Am J Pathol. 1996 Aug;149(2):559–574. [PMC free article] [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE ORIGIN OF MACROPHAGES FROM BONE MARROW IN THE RAT. Br J Exp Pathol. 1965 Feb;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- Van Zant G., Shultz L. Hematologic abnormalities of the immunodeficient mouse mutant, viable motheaten (mev). Exp Hematol. 1989 Feb;17(2):81–87. [PubMed] [Google Scholar]
- Wijffels J. F., Hendrickx R. J., Steenbergen J. J., Eestermans I. L., Beelen R. H. Milky spots in the mouse omentum may play an important role in the origin of peritoneal macrophages. Res Immunol. 1992 May;143(4):401–409. doi: 10.1016/s0923-2494(05)80072-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Zhu H., Naito M., Umezu H., Moriyama H., Takatsuka H., Takahashi K., Shultz L. D. Macrophage differentiation and expression of macrophage colony-stimulating factor in murine milky spots and omentum after macrophage elimination. J Leukoc Biol. 1997 Apr;61(4):436–444. doi: 10.1002/jlb.61.4.436. [DOI] [PubMed] [Google Scholar]