Abstract
Although a number of effective therapies are available for localized prostate cancer, metastatic prostate cancer is difficult to treat and impossible to cure. Identification of the gene products that enable a prostatic carcinoma cell to metastasize should facilitate an understanding of the processes leading to metastasis. To characterize the contribution of matrix metalloproteinase-9 (MMP-9, gelatinase B or the 92-kd type IV gelatinase/collagenase) to the development of metastasis in prostate cancer, we reduced MMP-9 expression in metastatic murine prostatic carcinoma cells using a ribozyme. The ribozyme transfected cells had lower basal levels of MMP-9 as well as decreased levels after stimulation by transforming growth factor-beta or phorbol 12-myristate 13-acetate when compared with the parental cells or with control transfectants. The cells with down-regulated MMP-9 were unable to form lung colonies in the experimental metastasis assay, whereas the controls and parental cells readily formed metastases. All cell types readily formed tumors after injection and down-regulation of MMP-9 did not adversely affect the rate of tumor growth. Thus, MMP-9 expression is required for hematogenous metastasis in a murine prostate model system raising the possibility that it may play an equivalent role in human prostate cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson S. J., Ward R. V., Reynolds J. J., Murphy G. Cell-mediated degradation of type IV collagen and gelatin films is dependent on the activation of matrix metalloproteinases. Biochem J. 1992 Dec 1;288(Pt 2):605–611. doi: 10.1042/bj2880605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard E. J., Gruber S. B., Muschel R. J. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4293–4297. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard E. J., Hagner B., Wong C., Lubenski I., Muschel R. J. The effect of E1A transfection on MMP-9 expression and metastatic potential. Int J Cancer. 1995 Mar 3;60(5):718–724. doi: 10.1002/ijc.2910600525. [DOI] [PubMed] [Google Scholar]
- Bernhard E. J., Muschel R. J., Hughes E. N. Mr 92,000 gelatinase release correlates with the metastatic phenotype in transformed rat embryo cells. Cancer Res. 1990 Jul 1;50(13):3872–3877. [PubMed] [Google Scholar]
- Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995 Oct;7(5):728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- DeClerck Y. A., Imren S. Protease inhibitors: role and potential therapeutic use in human cancer. Eur J Cancer. 1994;30A(14):2170–2180. doi: 10.1016/0959-8049(94)00460-m. [DOI] [PubMed] [Google Scholar]
- Desrivières S., Lu H., Peyri N., Soria C., Legrand Y., Ménashi S. Activation of the 92 kDa type IV collagenase by tissue kallikrein. J Cell Physiol. 1993 Dec;157(3):587–593. doi: 10.1002/jcp.1041570319. [DOI] [PubMed] [Google Scholar]
- Eklöv S., Funa K., Nordgren H., Olofsson A., Kanzaki T., Miyazono K., Nilsson S. Lack of the latent transforming growth factor beta binding protein in malignant, but not benign prostatic tissue. Cancer Res. 1993 Jul 1;53(13):3193–3197. [PubMed] [Google Scholar]
- Festuccia C., Bologna M., Vicentini C., Tacconelli A., Miano R., Violini S., Mackay A. R. Increased matrix metalloproteinase-9 secretion in short-term tissue cultures of prostatic tumor cells. Int J Cancer. 1996 Oct 21;69(5):386–393. doi: 10.1002/(SICI)1097-0215(19961021)69:5<386::AID-IJC6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Fridman R., Toth M., Peña D., Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res. 1995 Jun 15;55(12):2548–2555. [PubMed] [Google Scholar]
- Gearing A. J., Beckett P., Christodoulou M., Churchill M., Clements J. M., Crimmin M., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol. 1995 May;57(5):774–777. doi: 10.1002/jlb.57.5.774. [DOI] [PubMed] [Google Scholar]
- Glynne-Jones E., Harper M. E., Goddard L., Eaton C. L., Matthews P. N., Griffiths K. Transforming growth factor beta 1 expression in benign and malignant prostatic tumors. Prostate. 1994 Oct;25(4):210–218. doi: 10.1002/pros.2990250407. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- Greene J., Wang M., Liu Y. E., Raymond L. A., Rosen C., Shi Y. E. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996 Nov 29;271(48):30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- Hamdy F. C., Fadlon E. J., Cottam D., Lawry J., Thurrell W., Silcocks P. B., Anderson J. B., Williams J. L., Rees R. C. Matrix metalloproteinase 9 expression in primary human prostatic adenocarcinoma and benign prostatic hyperplasia. Br J Cancer. 1994 Jan;69(1):177–182. doi: 10.1038/bjc.1994.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E. W., Banda M. J. Binding of tissue inhibitor of metalloproteinases 2 to two distinct sites on human 72-kDa gelatinase. Identification of a stabilization site. J Biol Chem. 1991 Sep 25;266(27):17972–17977. [PubMed] [Google Scholar]
- Howard E. W., Bullen E. C., Banda M. J. Preferential inhibition of 72- and 92-kDa gelatinases by tissue inhibitor of metalloproteinases-2. J Biol Chem. 1991 Jul 15;266(20):13070–13075. [PubMed] [Google Scholar]
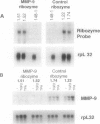
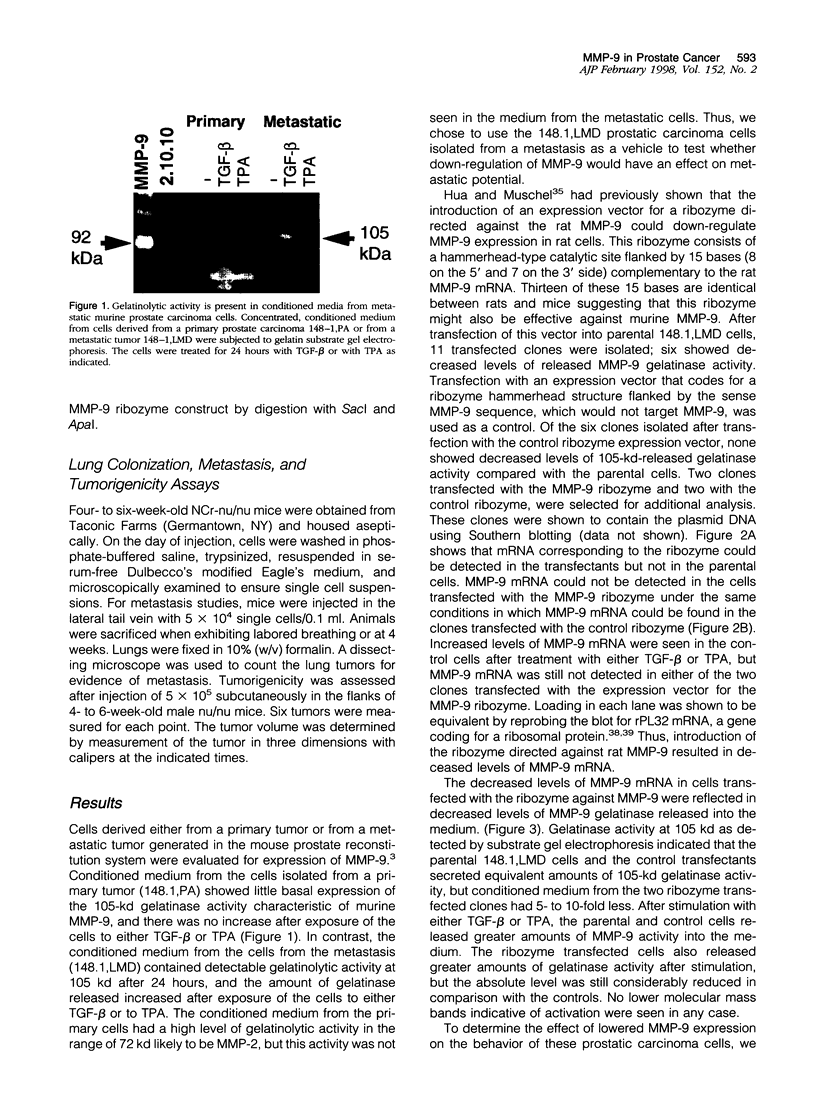
- Hua J., Muschel R. J. Inhibition of matrix metalloproteinase 9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996 Nov 15;56(22):5279–5284. [PubMed] [Google Scholar]
- Kim I. Y., Ahn H. J., Zelner D. J., Shaw J. W., Sensibar J. A., Kim J. H., Kato M., Lee C. Genetic change in transforming growth factor beta (TGF-beta) receptor type I gene correlates with insensitivity to TGF-beta 1 in human prostate cancer cells. Cancer Res. 1996 Jan 1;56(1):44–48. [PubMed] [Google Scholar]
- Leco K. J., Apte S. S., Taniguchi G. T., Hawkes S. P., Khokha R., Schultz G. A., Edwards D. R. Murine tissue inhibitor of metalloproteinases-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett. 1997 Jan 20;401(2-3):213–217. doi: 10.1016/s0014-5793(96)01474-3. [DOI] [PubMed] [Google Scholar]
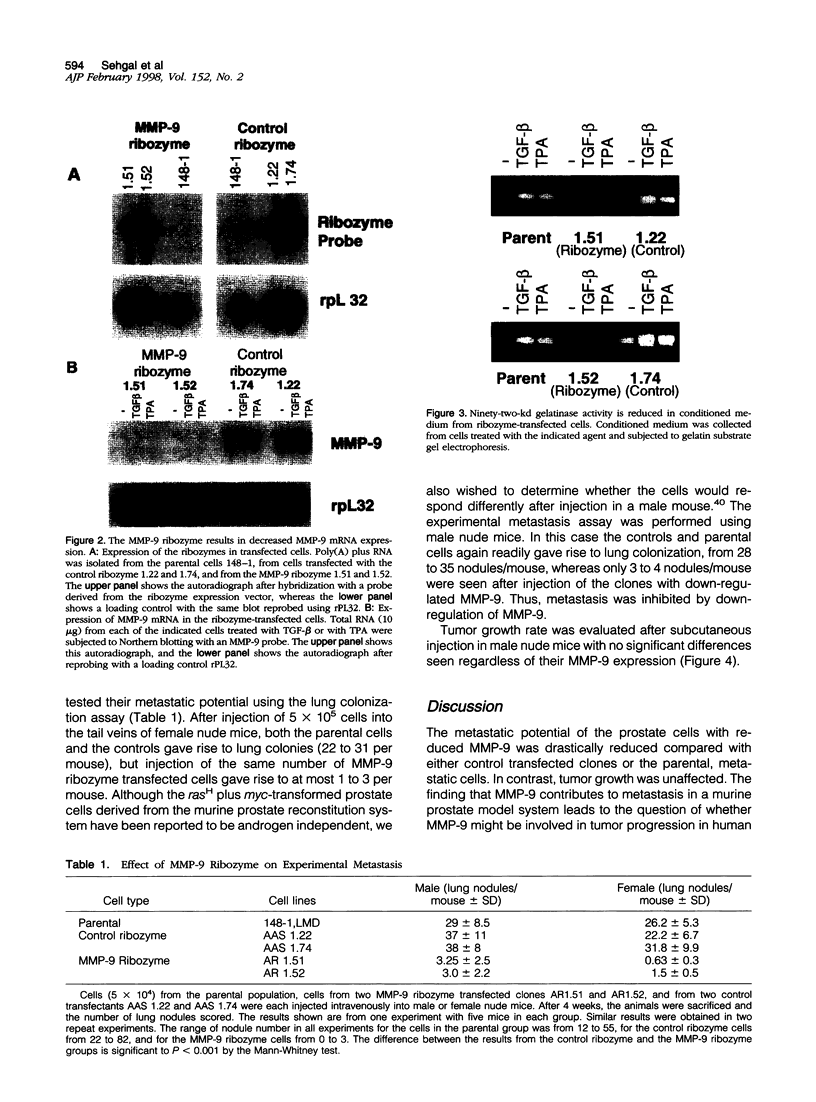
- Maity A., McKenna W. G., Muschel R. J. Evidence for post-transcriptional regulation of cyclin B1 mRNA in the cell cycle and following irradiation in HeLa cells. EMBO J. 1995 Feb 1;14(3):603–609. doi: 10.1002/j.1460-2075.1995.tb07036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz V. W., Arnold A. M., Studer U. E. Differential expression of transforming growth factor-beta 1 and beta 3 as well as c-fos mRNA in normal human prostate, benign prostatic hyperplasia and prostatic cancer. World J Urol. 1994;12(2):96–98. doi: 10.1007/BF00184244. [DOI] [PubMed] [Google Scholar]
- Merz V. W., Miller G. J., Krebs T., Timme T. L., Kadmon D., Park S. H., Egawa S., Scardino P. T., Thompson T. C. Elevated transforming growth factor-beta 1 and beta 3 mRNA levels are associated with ras + myc-induced carcinomas in reconstituted mouse prostate: evidence for a paracrine role during progression. Mol Endocrinol. 1991 Apr;5(4):503–513. doi: 10.1210/mend-5-4-503. [DOI] [PubMed] [Google Scholar]
- Meyuhas O., Perry R. P. Construction and identification of cDNA clones for mouse ribosomal proteins: application for the study of r-protein gene expression. Gene. 1980 Jul;10(2):113–129. doi: 10.1016/0378-1119(80)90129-8. [DOI] [PubMed] [Google Scholar]
- Murphy G., Willenbrock F. Tissue inhibitors of matrix metalloendopeptidases. Methods Enzymol. 1995;248:496–510. doi: 10.1016/0076-6879(95)48032-3. [DOI] [PubMed] [Google Scholar]
- Muschel R. J., Williams J. E., Lowy D. R., Liotta L. A. Harvey ras induction of metastatic potential depends upon oncogene activation and the type of recipient cell. Am J Pathol. 1985 Oct;121(1):1–8. [PMC free article] [PubMed] [Google Scholar]
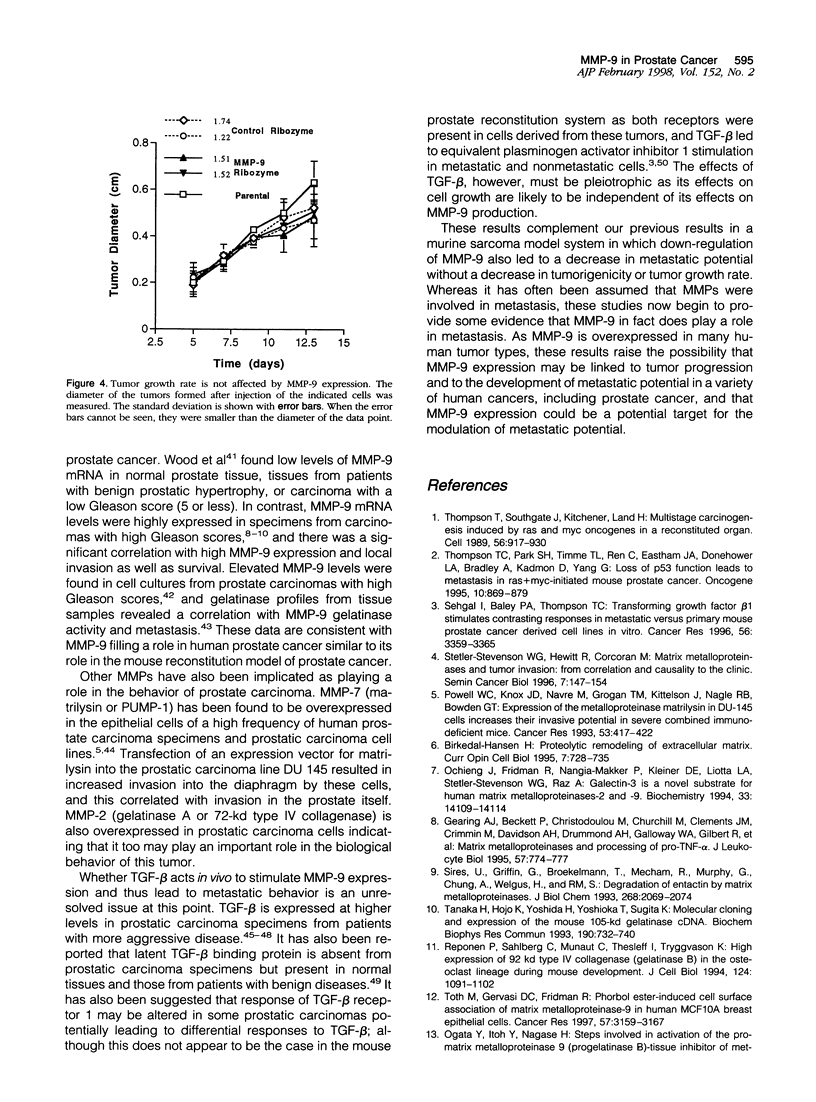
- O'Connell J. P., Willenbrock F., Docherty A. J., Eaton D., Murphy G. Analysis of the role of the COOH-terminal domain in the activation, proteolytic activity, and tissue inhibitor of metalloproteinase interactions of gelatinase B. J Biol Chem. 1994 May 27;269(21):14967–14973. [PubMed] [Google Scholar]
- Ochieng J., Fridman R., Nangia-Makker P., Kleiner D. E., Liotta L. A., Stetler-Stevenson W. G., Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994 Nov 29;33(47):14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- Ogata Y., Enghild J. J., Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992 Feb 25;267(6):3581–3584. [PubMed] [Google Scholar]
- Pajouh M. S., Nagle R. B., Breathnach R., Finch J. S., Brawer M. K., Bowden G. T. Expression of metalloproteinase genes in human prostate cancer. J Cancer Res Clin Oncol. 1991;117(2):144–150. doi: 10.1007/BF01613138. [DOI] [PubMed] [Google Scholar]
- Pavloff N., Staskus P. W., Kishnani N. S., Hawkes S. P. A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J Biol Chem. 1992 Aug 25;267(24):17321–17326. [PubMed] [Google Scholar]
- Powell W. C., Knox J. D., Navre M., Grogan T. M., Kittelson J., Nagle R. B., Bowden G. T. Expression of the metalloproteinase matrilysin in DU-145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res. 1993 Jan 15;53(2):417–422. [PubMed] [Google Scholar]
- Reponen P., Sahlberg C., Munaut C., Thesleff I., Tryggvason K. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994 Mar;124(6):1091–1102. doi: 10.1083/jcb.124.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Q. X., Birkedal-Hansen H., Van Wart H. E. Proteolytic and non-proteolytic activation of human neutrophil progelatinase B. Biochim Biophys Acta. 1995 Sep 6;1251(2):99–108. doi: 10.1016/0167-4838(95)00086-a. [DOI] [PubMed] [Google Scholar]
- Sehgal I., Baley P. A., Thompson T. C. Transforming growth factor beta1 stimulates contrasting responses in metastatic versus primary mouse prostate cancer-derived cell lines in vitro. Cancer Res. 1996 Jul 15;56(14):3359–3365. [PubMed] [Google Scholar]
- Shapiro S. D., Fliszar C. J., Broekelmann T. J., Mecham R. P., Senior R. M., Welgus H. G. Activation of the 92-kDa gelatinase by stromelysin and 4-aminophenylmercuric acetate. Differential processing and stabilization of the carboxyl-terminal domain by tissue inhibitor of metalloproteinases (TIMP). J Biol Chem. 1995 Mar 17;270(11):6351–6356. doi: 10.1074/jbc.270.11.6351. [DOI] [PubMed] [Google Scholar]
- Sires U. I., Griffin G. L., Broekelmann T. J., Mecham R. P., Murphy G., Chung A. E., Welgus H. G., Senior R. M. Degradation of entactin by matrix metalloproteinases. Susceptibility to matrilysin and identification of cleavage sites. J Biol Chem. 1993 Jan 25;268(3):2069–2074. [PubMed] [Google Scholar]
- Steiner M. S., Zhou Z. Z., Tonb D. C., Barrack E. R. Expression of transforming growth factor-beta 1 in prostate cancer. Endocrinology. 1994 Nov;135(5):2240–2247. doi: 10.1210/endo.135.5.7956947. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Hewitt R., Corcoran M. Matrix metalloproteinases and tumor invasion: from correlation and causality to the clinic. Semin Cancer Biol. 1996 Jun;7(3):147–154. doi: 10.1006/scbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Collier I. E., Krasnov P. A., Genrich L. T., Marmer B. L., Goldberg G. I. Human 92 kDa type IV collagenase: functional analysis of fibronectin and carboxyl-end domains. Kidney Int. 1993 Jan;43(1):158–162. doi: 10.1038/ki.1993.26. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Hojo K., Yoshida H., Yoshioka T., Sugita K. Molecular cloning and expression of the mouse 105-kDa gelatinase cDNA. Biochem Biophys Res Commun. 1993 Feb 15;190(3):732–740. doi: 10.1006/bbrc.1993.1110. [DOI] [PubMed] [Google Scholar]
- Thompson T. C., Egawa S., Kadmon D., Miller G. J., Timme T. L., Scardino P. T., Park S. H. Androgen sensitivity and gene expression in ras + myc-induced mouse prostate carcinomas. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):79–85. doi: 10.1016/0960-0760(92)90190-t. [DOI] [PubMed] [Google Scholar]
- Thompson T. C., Park S. H., Timme T. L., Ren C., Eastham J. A., Donehower L. A., Bradley A., Kadmon D., Yang G. Loss of p53 function leads to metastasis in ras+myc-initiated mouse prostate cancer. Oncogene. 1995 Mar 2;10(5):869–879. [PubMed] [Google Scholar]
- Thompson T. C., Southgate J., Kitchener G., Land H. Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell. 1989 Mar 24;56(6):917–930. doi: 10.1016/0092-8674(89)90625-9. [DOI] [PubMed] [Google Scholar]
- Toth M., Gervasi D. C., Fridman R. Phorbol ester-induced cell surface association of matrix metalloproteinase-9 in human MCF10A breast epithelial cells. Cancer Res. 1997 Aug 1;57(15):3159–3167. [PubMed] [Google Scholar]
- Willenbrock F., Crabbe T., Slocombe P. M., Sutton C. W., Docherty A. J., Cockett M. I., O'Shea M., Brocklehurst K., Phillips I. R., Murphy G. The activity of the tissue inhibitors of metalloproteinases is regulated by C-terminal domain interactions: a kinetic analysis of the inhibition of gelatinase A. Biochemistry. 1993 Apr 27;32(16):4330–4337. doi: 10.1021/bi00067a023. [DOI] [PubMed] [Google Scholar]
- Wood M., Fudge K., Mohler J. L., Frost A. R., Garcia F., Wang M., Stearns M. E. In situ hybridization studies of metalloproteinases 2 and 9 and TIMP-1 and TIMP-2 expression in human prostate cancer. Clin Exp Metastasis. 1997 May;15(3):246–258. doi: 10.1023/a:1018421431388. [DOI] [PubMed] [Google Scholar]
- Yang T. T., Hawkes S. P. Role of the 21-kDa protein TIMP-3 in oncogenic transformation of cultured chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10676–10680. doi: 10.1073/pnas.89.22.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Wang S. S., Dulski K. M., Tsai C. M., Nicolson G. L., Hung M. C. c-erbB-2/neu overexpression enhances metastatic potential of human lung cancer cells by induction of metastasis-associated properties. Cancer Res. 1994 Jun 15;54(12):3260–3266. [PubMed] [Google Scholar]