Abstract
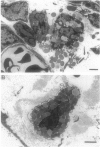
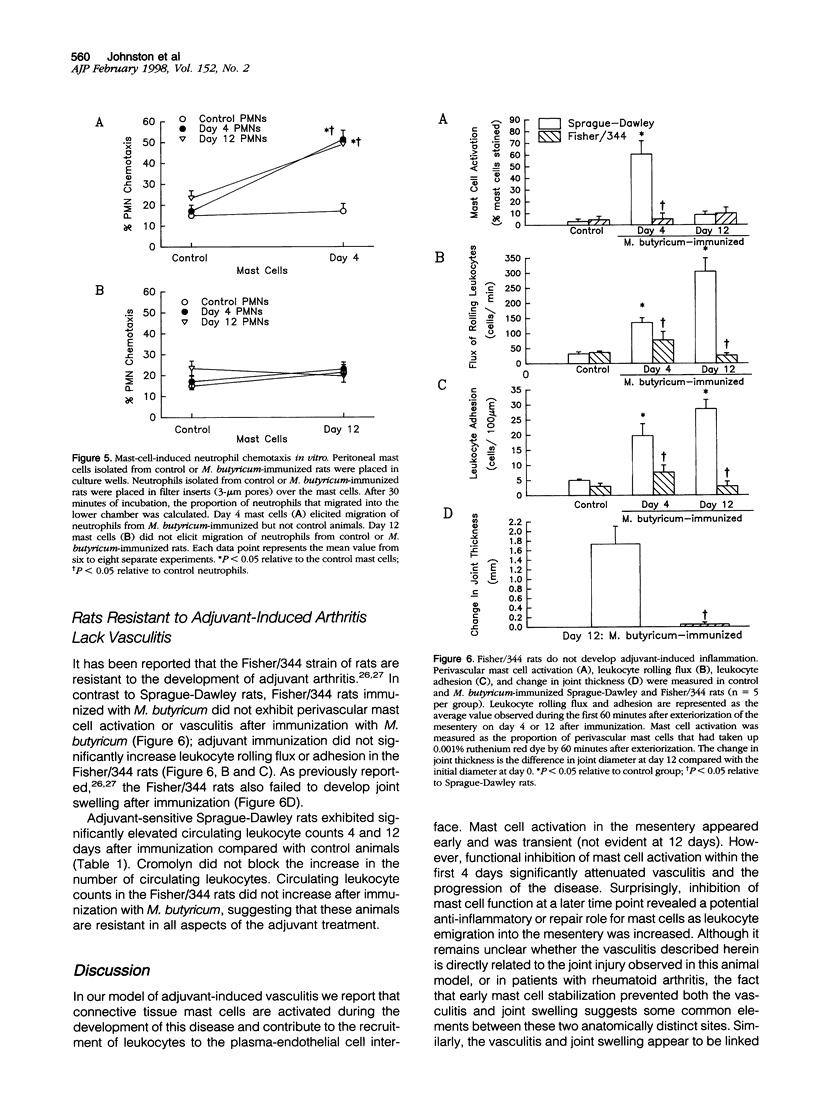
The objective of this study was to characterize the role of mast cells in the development of vasculitis and joint swelling in adjuvant-immunized rats. Leukocyte trafficking within mesenteric venules (rolling and adhesion) and mast cell activation (ruthenium red uptake) were examined in vivo. Elevated leukocyte trafficking was observed by 4 days after immunization, whereas joint swelling developed between days 10 and 12. Perivascular mast cells took up ruthenium red and appeared activated by electron microscopy at 4 but not 12 days after immunization. Treatment with the mast cell stabilizer cromolyn on days 1 to 4 after immunization blocked ruthenium red uptake at day 4 and reduced leukocyte rolling and adhesion by approximately 50%. This treatment also reduced rolling, adhesion, and joint swelling at day 12 by approximately 50%. Cromolyn treatment over days 9 to 12 reduced joint swelling but increased leukocyte emigration into the mesentery. Peritoneal mast cells isolated 4 days after immunization elicited significant neutrophil chemotaxis in vitro, whereas day 12 mast cells did not. Mast cell activation and vasculitis were absent in adjuvant-resistant Fisher/344 rats. These data suggest that mast cells play an early role in the initiation of vasculitis and may function by day 12 to limit infiltration of leukocytes from the vasculature. In the joint, however, mast cells appear to contribute to inflammation at early as well as later time points.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges A. J., Malone D. G., Jicinsky J., Chen M., Ory P., Engber W., Graziano F. M. Human synovial mast cell involvement in rheumatoid arthritis and osteoarthritis. Relationship to disease type, clinical activity, and antirheumatic therapy. Arthritis Rheum. 1991 Sep;34(9):1116–1124. doi: 10.1002/art.1780340907. [DOI] [PubMed] [Google Scholar]
- Burns A. R., Simon S. I., Kukielka G. L., Rowen J. L., Lu H., Mendoza L. H., Brown E. S., Entman M. L., Smith C. W. Chemotactic factors stimulate CD18-dependent canine neutrophil adherence and motility on lung fibroblasts. J Immunol. 1996 May 1;156(9):3389–3401. [PubMed] [Google Scholar]
- Caulfield J. P., Hein A., Helfgott S. M., Brahn E., Dynesius-Trentham R. A., Trentham D. E. Intraarticular injection of arthritogenic factor causes mast cell degranulation, inflammation, fat necrosis, and synovial hyperplasia. Lab Invest. 1988 Jul;59(1):82–95. [PubMed] [Google Scholar]
- Crisp A. J., Chapman C. M., Kirkham S. E., Schiller A. L., Krane S. M. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984 Aug;27(8):845–851. doi: 10.1002/art.1780270802. [DOI] [PubMed] [Google Scholar]
- Crowe S. E., Perdue M. H. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterology. 1992 Sep;103(3):1075–1095. doi: 10.1016/0016-5085(92)90047-3. [DOI] [PubMed] [Google Scholar]
- Currey H. L., Ziff M. Suppression of adjuvant disease in the rat by heterologous antilymphocyte globulin. J Exp Med. 1968 Jan 1;127(1):185–203. doi: 10.1084/jem.127.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Anderle S. K., Brown R. R., Schwab J. H. Mast cell activation by group A streptococcal polysaccharide in the rat and its role in experimental arthritis. Am J Pathol. 1988 Aug;132(2):258–264. [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Echtenacher B., Männel D. N., Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996 May 2;381(6577):75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Fox C. C., Jewell S. D., Whitacre C. C. Rat peritoneal mast cells present antigen to a PPD-specific T cell line. Cell Immunol. 1994 Oct 1;158(1):253–264. doi: 10.1006/cimm.1994.1272. [DOI] [PubMed] [Google Scholar]
- Gaboury J. P., Johnston B., Niu X. F., Kubes P. Mechanisms underlying acute mast cell-induced leukocyte rolling and adhesion in vivo. J Immunol. 1995 Jan 15;154(2):804–813. [PubMed] [Google Scholar]
- Gaboury J. P., Niu X. F., Kubes P. Nitric oxide inhibits numerous features of mast cell-induced inflammation. Circulation. 1996 Jan 15;93(2):318–326. doi: 10.1161/01.cir.93.2.318. [DOI] [PubMed] [Google Scholar]
- Galli S. J. New concepts about the mast cell. N Engl J Med. 1993 Jan 28;328(4):257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Burd P. R., Galli S. J. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990 Dec;11(12):458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- Gryfe A., Sanders P. M., Gardner D. L. The mast cell in early rat adjuvant arthritis. Ann Rheum Dis. 1971 Jan;30(1):24–30. doi: 10.1136/ard.30.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam C. M., Befus A. D., Wallace J. L. Modulation of rat mast cell reactivity by IL-1 beta. Divergent effects on nitric oxide and platelet-activating factor release. J Immunol. 1993 Oct 1;151(7):3767–3774. [PubMed] [Google Scholar]
- Hogervorst E. J., Boog C. J., Wagenaar J. P., Wauben M. H., Van der Zee R., Van Eden W. T cell reactivity to an epitope of the mycobacterial 65-kDa heat-shock protein (hsp 65) corresponds with arthritis susceptibility in rats and is regulated by hsp 65-specific cellular responses. Eur J Immunol. 1991 May;21(5):1289–1296. doi: 10.1002/eji.1830210529. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Issekutz A. C. T lymphocyte migration to arthritic joints and dermal inflammation in the rat: differing migration patterns and the involvement of VLA-4. Clin Immunol Immunopathol. 1991 Dec;61(3):436–447. doi: 10.1016/s0090-1229(05)80014-5. [DOI] [PubMed] [Google Scholar]
- Johnston B., Walter U. M., Issekutz A. C., Issekutz T. B., Anderson D. C., Kubes P. Differential roles of selectins and the alpha4-integrin in acute, subacute, and chronic leukocyte recruitment in vivo. J Immunol. 1997 Nov 1;159(9):4514–4523. [PubMed] [Google Scholar]
- Kanwar S., Kubes P. Ischemia/reperfusion-induced granulocyte influx is a multistep process mediated by mast cells. Microcirculation. 1994 Oct;1(3):175–182. doi: 10.3109/10739689409148272. [DOI] [PubMed] [Google Scholar]
- Klein L. M., Lavker R. M., Matis W. L., Murphy G. F. Degranulation of human mast cells induces an endothelial antigen central to leukocyte adhesion. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8972–8976. doi: 10.1073/pnas.86.22.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Leukocyte-endothelial cell interactions evoked by mast cells. Cardiovasc Res. 1996 Oct;32(4):699–708. [PubMed] [Google Scholar]
- Kubes P., Kanwar S. Histamine induces leukocyte rolling in post-capillary venules. A P-selectin-mediated event. J Immunol. 1994 Apr 1;152(7):3570–3577. [PubMed] [Google Scholar]
- Lagunoff D. Vital staining of mast cells with ruthenium red. J Histochem Cytochem. 1972 Nov;20(11):938–944. doi: 10.1177/20.11.938. [DOI] [PubMed] [Google Scholar]
- Lioté F., Boval-Boizard B., Weill D., Kuntz D., Wautier J. L. Blood monocyte activation in rheumatoid arthritis: increased monocyte adhesiveness, integrin expression, and cytokine release. Clin Exp Immunol. 1996 Oct;106(1):13–19. doi: 10.1046/j.1365-2249.1996.d01-820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance J. H., O'Sullivan F. X., Caver T. E., Waegell W., Gresham H. D. Spontaneous elaboration of transforming growth factor beta suppresses host defense against bacterial infection in autoimmune MRL/lpr mice. J Exp Med. 1994 Nov 1;180(5):1693–1703. doi: 10.1084/jem.180.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R., Ikeda T., Ross E., Abraham S. N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996 May 2;381(6577):77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Marietta E. V., Chen Y., Weis J. H. Modulation of expression of the anti-inflammatory cytokines interleukin-13 and interleukin-10 by interleukin-3. Eur J Immunol. 1996 Jan;26(1):49–56. doi: 10.1002/eji.1830260108. [DOI] [PubMed] [Google Scholar]
- McCafferty D. M., Granger D. N., Wallace J. L. Indomethacin-induced gastric injury and leukocyte adherence in arthritic versus healthy rats. Gastroenterology. 1995 Oct;109(4):1173–1180. doi: 10.1016/0016-5085(95)90576-6. [DOI] [PubMed] [Google Scholar]
- Meng H., Tonnesen M. G., Marchese M. J., Clark R. A., Bahou W. F., Gruber B. L. Mast cells are potent regulators of endothelial cell adhesion molecule ICAM-1 and VCAM-1 expression. J Cell Physiol. 1995 Oct;165(1):40–53. doi: 10.1002/jcp.1041650106. [DOI] [PubMed] [Google Scholar]
- Nelson R. M., Cecconi O., Roberts W. G., Aruffo A., Linhardt R. J., Bevilacqua M. P. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993 Dec 1;82(11):3253–3258. [PubMed] [Google Scholar]
- Niu X. F., Smith C. W., Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res. 1994 Jun;74(6):1133–1140. doi: 10.1161/01.res.74.6.1133. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M., WAKSMAN B. H., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. V. Changes affecting the skin and mucous membranes. Comparison of the experimental process with human disease. J Exp Med. 1961 Mar 1;113:485–510. doi: 10.1084/jem.113.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrí C., Paz Morante M., Castellote C., Castell M., Franch A. Administration of a nondepleting anti-CD4 monoclonal antibody (W3/25) prevents adjuvant arthritis, even upon rechallenge: parallel administration of a depleting anti-CD8 monoclonal antibody (OX8) does not modify the effect of W3/25. Cell Immunol. 1995 Oct 15;165(2):177–182. doi: 10.1006/cimm.1995.1203. [DOI] [PubMed] [Google Scholar]
- Raud J., Lindbom L., Dahlèn S. E., Hedqvist P. Periarteriolar localization of mast cells promotes oriented interstitial migration of leukocytes in the hamster cheek pouch. Am J Pathol. 1989 Jan;134(1):161–169. [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Masini E., Anggard E., Mannaioni P. F., Vane J. Synthesis of a nitric oxide-like factor from L-arginine by rat serosal mast cells: stimulation of guanylate cyclase and inhibition of platelet aggregation. Biochem Biophys Res Commun. 1990 Jun 15;169(2):596–601. doi: 10.1016/0006-291x(90)90372-t. [DOI] [PubMed] [Google Scholar]
- Sfikakis P. P., Tsokos G. C. Lymphocyte adhesion molecules in autoimmune rheumatic diseases: basic issues and clinical expectations. Clin Exp Rheumatol. 1995 Nov-Dec;13(6):763–777. [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985 Aug;135(2):1331–1337. [PubMed] [Google Scholar]
- Swain M. G., Maric M. Prevention of immune-mediated arthritis in cholestatic rats: involvement of endogenous glucocorticoids. Gastroenterology. 1994 Nov;107(5):1469–1474. doi: 10.1016/0016-5085(94)90551-7. [DOI] [PubMed] [Google Scholar]
- Takagi M., Nakahata T., Koike K., Kobayashi T., Tsuji K., Kojima S., Hirano T., Miyajima A., Arai K., Akabane T. Stimulation of connective tissue-type mast cell proliferation by crosslinking of cell-bound IgE. J Exp Med. 1989 Jul 1;170(1):233–244. doi: 10.1084/jem.170.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum S., Oertel H., Henderson W., Kaliner M. The biologic activity of mast cell granules. I. Elicitation of inflammatory responses in rat skin. J Immunol. 1980 Jul;125(1):325–335. [PubMed] [Google Scholar]
- Van Arman C. G. Pathway to adjuvant arthritis. Fed Proc. 1976 Nov;35(13):2442–2446. [PubMed] [Google Scholar]
- WAKSMAN B. H., PEARSON C. M., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. J Immunol. 1960 Oct;85:403–417. [PubMed] [Google Scholar]
- Wasserman S. I. The mast cell and synovial inflammation. Or, what's a nice cell like you doing in a joint like this? Arthritis Rheum. 1984 Aug;27(8):841–844. doi: 10.1002/art.1780270801. [DOI] [PubMed] [Google Scholar]
- Wershil B. K., Wang Z. S., Gordon J. R., Galli S. J. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991 Feb;87(2):446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. M., Coleman J. W. Induced expression of mRNA for IL-5, IL-6, TNF-alpha, MIP-2 and IFN-gamma in immunologically activated rat peritoneal mast cells: inhibition by dexamethasone and cyclosporin A. Immunology. 1995 Oct;86(2):244–249. [PMC free article] [PubMed] [Google Scholar]
- Woodman R. C., Reinhardt P. H., Kanwar S., Johnston F. L., Kubes P. Effects of human neutrophil elastase (HNE) on neutrophil function in vitro and in inflamed microvessels. Blood. 1993 Oct 1;82(7):2188–2195. [PubMed] [Google Scholar]
- Yoshino S., Cleland L. G. Depletion of alpha/beta T cells by a monoclonal antibody against the alpha/beta T cell receptor suppresses established adjuvant arthritis, but not established collagen-induced arthritis in rats. J Exp Med. 1992 Apr 1;175(4):907–915. doi: 10.1084/jem.175.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Clerck L. S., Struyf N. J., Bridts C. H., van Marck E. A., Breedveld F. C., Devries E., Bazin H., Stevens W. J. Experimental arthritis in rats induced by intra-articular injection of IgE aggregates: evidence for arthritogenic role of complexed IgE. Ann Rheum Dis. 1992 Feb;51(2):210–213. doi: 10.1136/ard.51.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Langerijt A. G., van Lent P. L., Hermus A. R., van de Putte L. B., van den Berg W. B. Regulation of resistance against adjuvant arthritis in the Fisher rat. Clin Exp Immunol. 1993 Oct;94(1):150–155. doi: 10.1111/j.1365-2249.1993.tb05993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M. F., van den Berg W. B., van de Putte L. B. The role of mast cells in antigen induced arthritis in mice. J Rheumatol. 1988 Apr;15(4):544–551. [PubMed] [Google Scholar]