Abstract
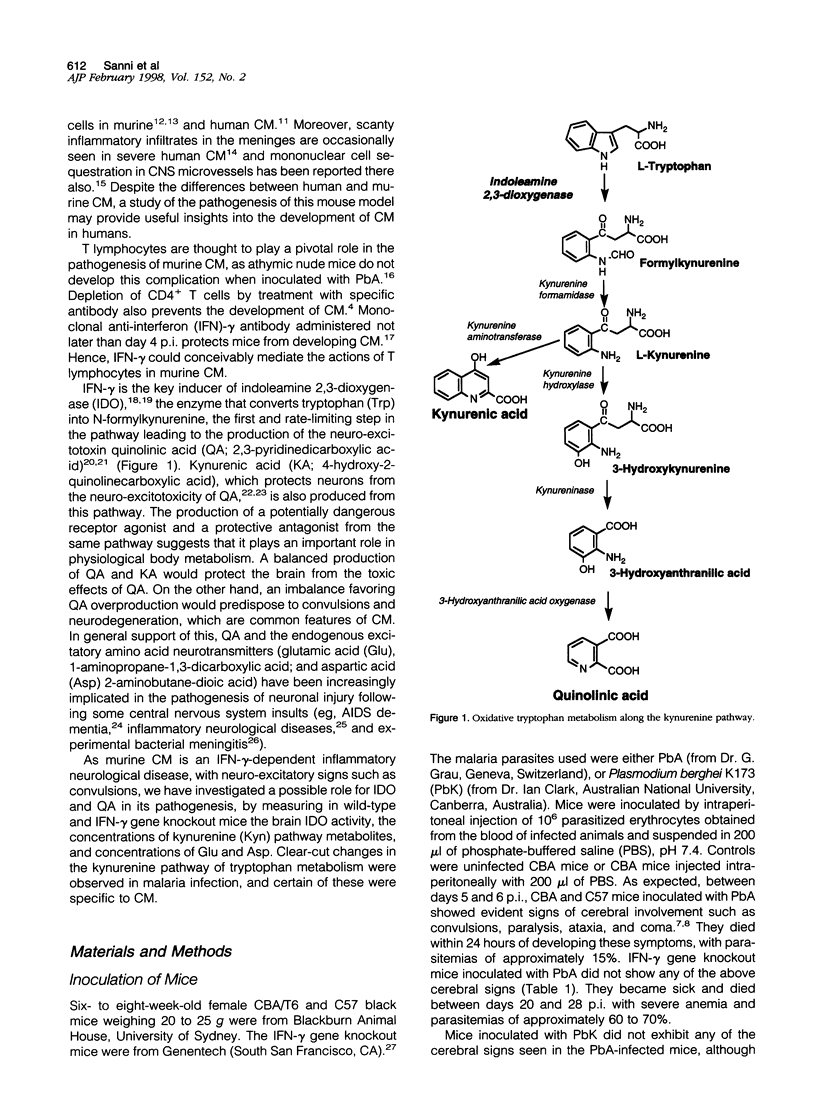
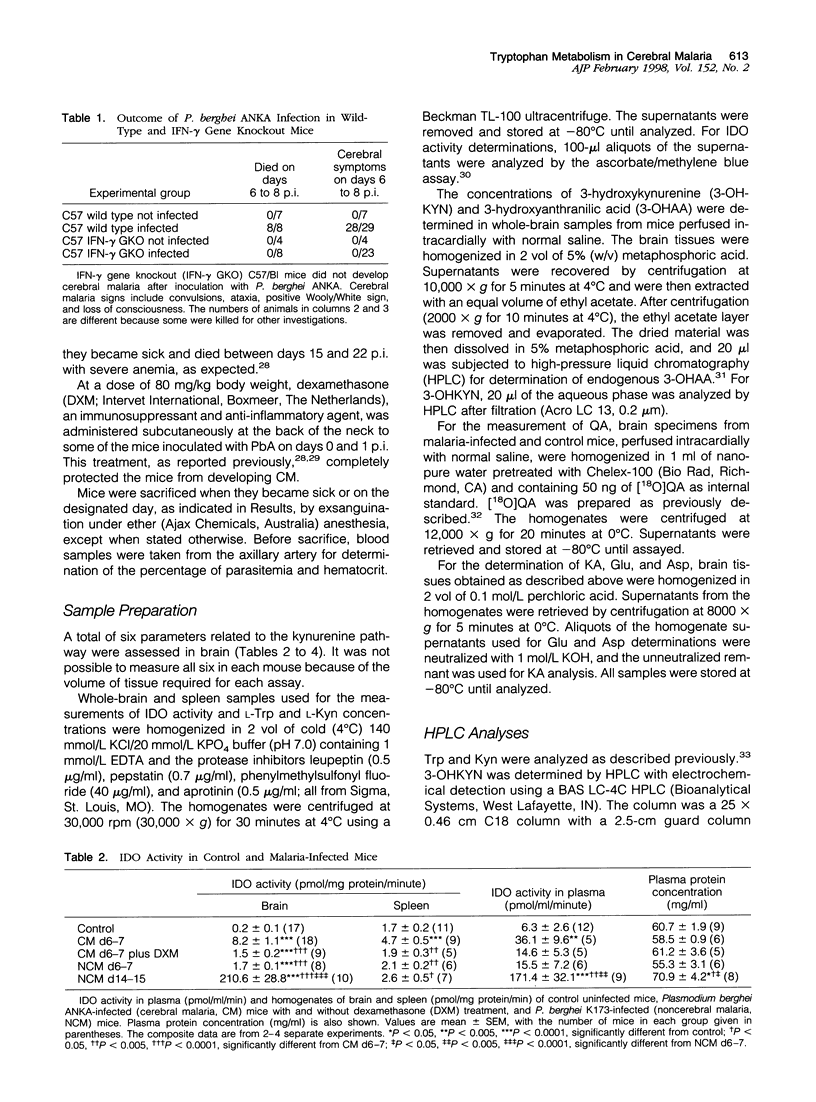
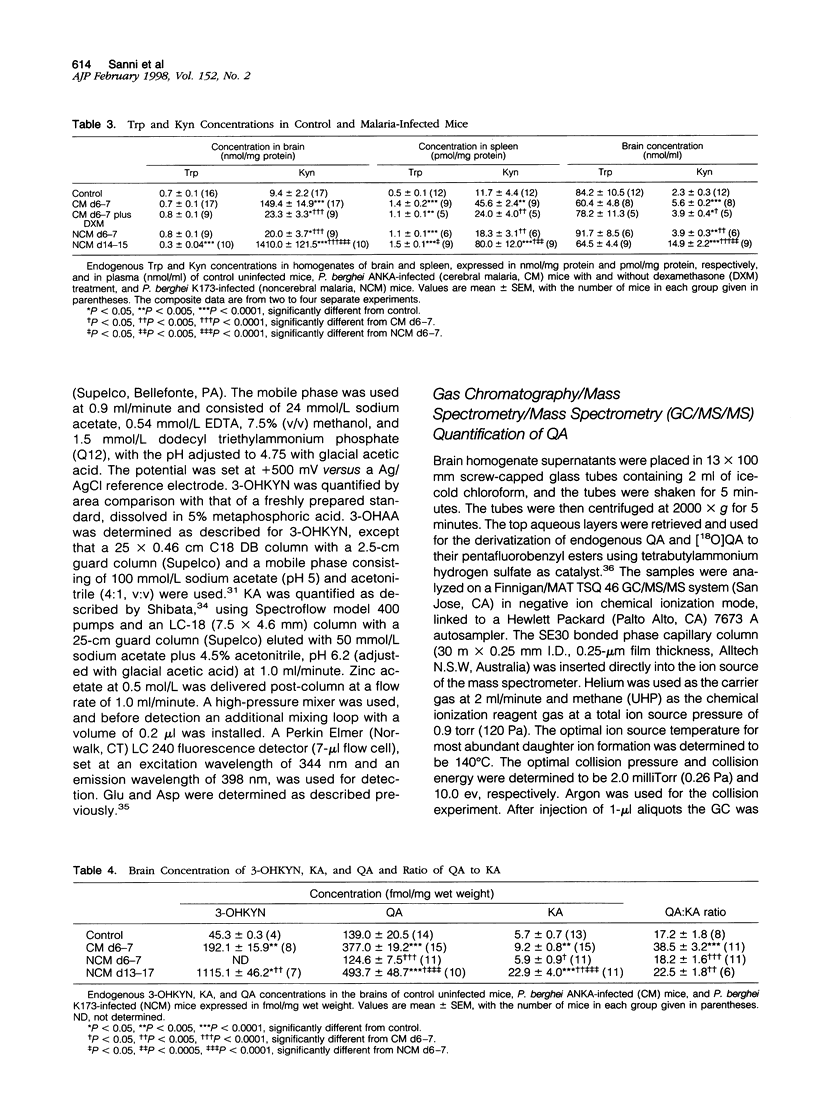
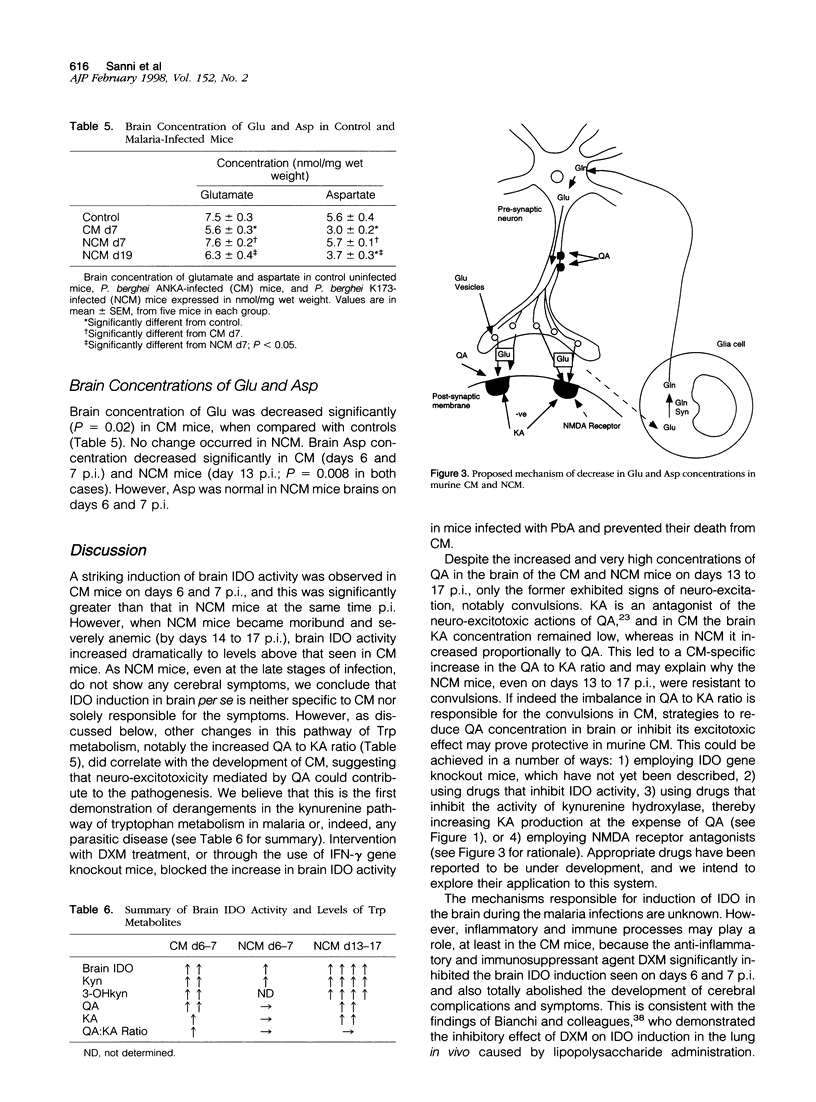
The pathogenesis of human cerebral malaria (CM) remains unresolved. In the most widely used murine model of CM, the presence of T lymphocytes and/or interferon (IFN)-gamma is a prerequisite. IFN-gamma is the key inducer of indoleamine 2,3-dioxygenase (IDO), which is the catalyst of the first, and rate-limiting, step in the metabolism of tryptophan (Trp) along the kynurenine (Kyn) pathway. Quinolinic acid (QA), a product of this pathway, is a neuro-excitotoxin, like glutamic acid (Glu) and aspartic acid (Asp). Kynurenic acid (KA), also produced from the Kyn pathway, antagonizes the neuro-excitotoxic effects of QA, Glu, and Asp. We therefore examined the possible roles of IDO, metabolites of the Kyn pathway, Glu, and Asp in the pathogenesis of fatal murine CM. Plasmodium berghei ANKA infection was studied on days 6 and 7 post-inoculation (p.i.), at which time the mice exhibited cerebral symptoms such as convulsions, ataxia, coma, and a positive Wooly/White sign and died within 24 hours. A model for noncerebral malaria (NCM), P. berghei K173 infection, was also studied on days 6 and 7 and 13 to 17 p.i. to examine whether any changes were a general response to malaria infection. Biochemical analyses were done by high-pressure liquid chromatography and gas chromatography/mass spectrometry/mass spectrometry (GC/MS/MS). IDO activity was low or absent in the brains of uninfected mice and NCM mice (days 6 and 7 p.i.) and was induced strongly in the brains of fatal murine CM mice (days 6 and 7 p.i.) and NCM animals (days 13 to 17 p.i.). This induction was inhibited greatly by administration of dexamethasone, a treatment that also prevented CM symptoms and death. Furthermore, IDO induction was absent in IFN-gamma gene knockout mice, which were also resistant to CM. Brain concentrations of Kyn, 3-hydroxykynurenine, and the neuro-excitotoxin QA were significantly increased in both CM mice on days 6 and 7 p.i. and NCM mice on days 13 to 17 p.i., whereas an increase in the ratio of brain QA to KA occurred only in the CM mice at the time they were exhibiting cerebral symptoms. Brain concentrations of Glu and Asp were significantly decreased in CM and NCM mice (days 13 to 17 p.i.). The results imply that neuro-excitation induced by QA may contribute to the convulsions and neuro-excitatory signs observed in CM.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Iseki M., Barnwell J. W., Taylor D., Oo M. M., Howard R. J. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990 Aug;43(2 Pt 2):30–37. doi: 10.4269/ajtmh.1990.43.30. [DOI] [PubMed] [Google Scholar]
- Alberati-Giani D., Ricciardi-Castagnoli P., Köhler C., Cesura A. M. Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells. J Neurochem. 1996 Mar;66(3):996–1004. doi: 10.1046/j.1471-4159.1996.66030996.x. [DOI] [PubMed] [Google Scholar]
- Baethmann A., Maier-Hauff K., Schürer L., Lange M., Guggenbichler C., Vogt W., Jacob K., Kempski O. Release of glutamate and of free fatty acids in vasogenic brain edema. J Neurosurg. 1989 Apr;70(4):578–591. doi: 10.3171/jns.1989.70.4.0578. [DOI] [PubMed] [Google Scholar]
- Bianchi M., Bertini R., Ghezzi P. Induction of indoleamine dioxygenase by interferon in mice: a study with different recombinant interferons and various cytokines. Biochem Biophys Res Commun. 1988 Apr 15;152(1):237–242. doi: 10.1016/s0006-291x(88)80705-8. [DOI] [PubMed] [Google Scholar]
- Chang-Ling T., Neill A. L., Hunt N. H. Early microvascular changes in murine cerebral malaria detected in retinal wholemounts. Am J Pathol. 1992 May;140(5):1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Christen S., Stocker R. Simultaneous determination of 3-hydroxyanthranilic and cinnabarinic acid by high-performance liquid chromatography with photometric or electrochemical detection. Anal Biochem. 1992 Feb 1;200(2):273–279. doi: 10.1016/0003-2697(92)90465-j. [DOI] [PubMed] [Google Scholar]
- Christen S., Thomas S. R., Garner B., Stocker R. Inhibition by interferon-gamma of human mononuclear cell-mediated low density lipoprotein oxidation. Participation of tryptophan metabolism along the kynurenine pathway. J Clin Invest. 1994 May;93(5):2149–2158. doi: 10.1172/JCI117211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick J. H., Stone T. W. Quinolinic acid effects on amino acid release from the rat cerebral cortex in vitro and in vivo. Br J Pharmacol. 1988 Apr;93(4):868–876. doi: 10.1111/j.1476-5381.1988.tb11474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Drejer J., Larsson O. M., Schousboe A. Characterization of L-glutamate uptake into and release from astrocytes and neurons cultured from different brain regions. Exp Brain Res. 1982;47(2):259–269. doi: 10.1007/BF00239385. [DOI] [PubMed] [Google Scholar]
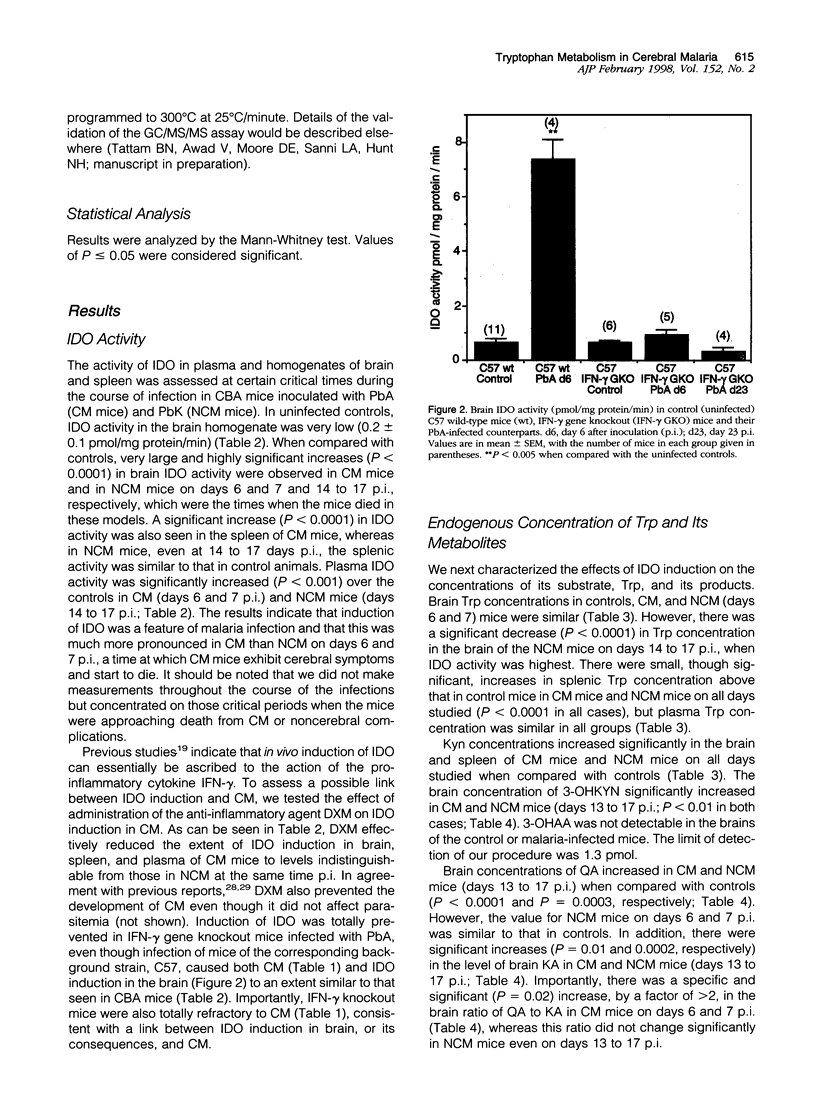
- Elesha S. O., Adepoju F. B., Banjo A. A. Rising incidence of cerebral malaria in Lagos, Nigeria: a postmoterm study. East Afr Med J. 1993 May;70(5):302–306. [PubMed] [Google Scholar]
- Espey M. G., Tang Y., Morse H. C., 3rd, Moffett J. R., Namboodiri M. A. Localization of quinolinic acid in the murine AIDS model of retrovirus-induced immunodeficiency: implications for neurotoxicity and dendritic cell immunopathogenesis. AIDS. 1996 Feb;10(2):151–158. doi: 10.1097/00002030-199602000-00004. [DOI] [PubMed] [Google Scholar]
- Finley R. W., Mackey L. J., Lambert P. H. Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in cell-dependent nude mice. J Immunol. 1982 Nov;129(5):2213–2218. [PubMed] [Google Scholar]
- Foster A. C., Vezzani A., French E. D., Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. 1984 Aug 10;48(3):273–278. doi: 10.1016/0304-3940(84)90050-8. [DOI] [PubMed] [Google Scholar]
- Fukui S., Schwarcz R., Rapoport S. I., Takada Y., Smith Q. R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991 Jun;56(6):2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Heremans H., Piguet P. F., Pointaire P., Lambert P. H., Billiau A., Vassalli P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Grau G. E., Pointaire P., Piguet P. F., Vesin C., Rosen H., Stamenkovic I., Takei F., Vassalli P. Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. Eur J Immunol. 1991 Sep;21(9):2265–2267. doi: 10.1002/eji.1830210939. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Brew B. J., Martin A., Price R. W., Salazar A. M., Sidtis J. J., Yergey J. A., Mouradian M. M., Sadler A. E., Keilp J. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991 Feb;29(2):202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Lackner A. Increased cerebrospinal fluid quinolinic acid, kynurenic acid, and L-kynurenine in acute septicemia. J Neurochem. 1990 Jul;55(1):338–341. doi: 10.1111/j.1471-4159.1990.tb08857.x. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Markey S. P. (18O)quinolinic acid: its esterification without back exchange for use as internal standard in the quantification of brain and CSF quinolinic acid. Biomed Environ Mass Spectrom. 1988 Mar 1;15(5):291–293. doi: 10.1002/bms.1200150509. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Saito K., Crowley J. S., Davis L. E., Demitrack M. A., Der M., Dilling L. A., Elia J., Kruesi M. J., Lackner A. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992 Oct;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- Jacobs P., Radzioch D., Stevenson M. M. Nitric oxide expression in the spleen, but not in the liver, correlates with resistance to blood-stage malaria in mice. J Immunol. 1995 Dec 1;155(11):5306–5313. [PubMed] [Google Scholar]
- Leib S. L., Kim Y. S., Ferriero D. M., Täuber M. G. Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J Infect Dis. 1996 Jan;173(1):166–171. doi: 10.1093/infdis/173.1.166. [DOI] [PubMed] [Google Scholar]
- Ma N., Hunt N. H., Madigan M. C., Chan-Ling T. Correlation between enhanced vascular permeability, up-regulation of cellular adhesion molecules and monocyte adhesion to the endothelium in the retina during the development of fatal murine cerebral malaria. Am J Pathol. 1996 Nov;149(5):1745–1762. [PMC free article] [PubMed] [Google Scholar]
- Medana I. M., Chan-Ling T., Hunt N. H. Redistribution and degeneration of retinal astrocytes in experimental murine cerebral malaria: relationship to disruption of the blood-retinal barrier. Glia. 1996 Jan;16(1):51–64. doi: 10.1002/(SICI)1098-1136(199601)16:1<51::AID-GLIA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Medana I. M., Hunt N. H., Chan-Ling T. Early activation of microglia in the pathogenesis of fatal murine cerebral malaria. Glia. 1997 Feb;19(2):91–103. doi: 10.1002/(sici)1098-1136(199702)19:2<91::aid-glia1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Naritsin D. B., Boni R. L., Markey S. P. Pentafluorobenzylation method for quantification of acidic tryptophan metabolites using electron capture negative ion mass spectrometry. Anal Chem. 1995 Mar 1;67(5):863–870. doi: 10.1021/ac00101a012. [DOI] [PubMed] [Google Scholar]
- Neill A. L., Hunt N. H. Effects of endotoxin and dexamethasone on cerebral malaria in mice. Parasitology. 1995 Nov;111(Pt 4):443–454. doi: 10.1017/s003118200006594x. [DOI] [PubMed] [Google Scholar]
- Neill A. L., Hunt N. H. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology. 1992 Oct;105(Pt 2):165–175. doi: 10.1017/s0031182000074072. [DOI] [PubMed] [Google Scholar]
- Neuzil J., Gebicki J. M., Stocker R. Radical-induced chain oxidation of proteins and its inhibition by chain-breaking antioxidants. Biochem J. 1993 Aug 1;293(Pt 3):601–606. doi: 10.1042/bj2930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik J. K., Das B. S., Mishra S. K., Mohanty S., Satpathy S. K., Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994 Nov;51(5):642–647. [PubMed] [Google Scholar]
- Perkins M. N., Stone T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982 Sep 9;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Rest J. R. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76(3):410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Román G. C. Cerebral malaria: the unsolved riddle. J Neurol Sci. 1991 Jan;101(1):1–6. doi: 10.1016/0022-510x(91)90012-v. [DOI] [PubMed] [Google Scholar]
- Saito K., Markey S. P., Heyes M. P. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992 Nov;51(1):25–39. doi: 10.1016/0306-4522(92)90467-g. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Shibata K. Fluorimetric micro-determination of kynurenic acid, an endogenous blocker of neurotoxicity, by high-performance liquid chromatography. J Chromatogr. 1988 Sep 9;430(2):376–380. doi: 10.1016/s0378-4347(00)83173-4. [DOI] [PubMed] [Google Scholar]
- Sono M. The roles of superoxide anion and methylene blue in the reductive activation of indoleamine 2,3-dioxygenase by ascorbic acid or by xanthine oxidase-hypoxanthine. J Biol Chem. 1989 Jan 25;264(3):1616–1622. [PubMed] [Google Scholar]
- Stone T. W. Differences of neuronal sensitivity to amino acids and related compounds in the rat hippocampal slice. Neurosci Lett. 1985 Sep 6;59(3):313–317. doi: 10.1016/0304-3940(85)90151-x. [DOI] [PubMed] [Google Scholar]
- Thomas S. R., Mohr D., Stocker R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J Biol Chem. 1994 May 20;269(20):14457–14464. [PubMed] [Google Scholar]
- Thumwood C. M., Hunt N. H., Clark I. A., Cowden W. B. Breakdown of the blood-brain barrier in murine cerebral malaria. Parasitology. 1988 Jun;96(Pt 3):579–589. doi: 10.1017/s0031182000080203. [DOI] [PubMed] [Google Scholar]
- Walker O., Salako L. A., Sowunmi A., Thomas J. O., Sodeine O., Bondi F. S. Prognostic risk factors and post mortem findings in cerebral malaria in children. Trans R Soc Trop Med Hyg. 1992 Sep-Oct;86(5):491–493. doi: 10.1016/0035-9203(92)90082-n. [DOI] [PubMed] [Google Scholar]
- Wyler D. J. Malaria--resurgence, resistance, and research. (First of two parts). N Engl J Med. 1983 Apr 14;308(15):875–878. doi: 10.1056/NEJM198304143081505. [DOI] [PubMed] [Google Scholar]


