Abstract
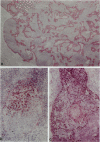
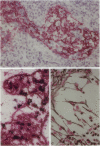
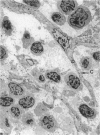
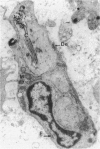
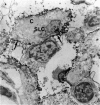
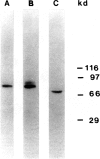
In the immunobiological characterization of lymph node cells, sinus-lining cells (SLCs) have been given little attention mainly due to the difficulties in their recognition. Ki-M9 is a new monoclonal antibody (MAb) selected for its unique capability to visualize SLCs in human lymph nodes. The details were established by light and electron microscopy and immunoprecipitation of the corresponding biosynthetically labeled antigen. Ki-M9 recognizes a 70-kd protein localized on the surface membrane of SLCs. In the lymphoid tissue, a mild reactivity was exclusively encountered on follicular dendritic reticulum cells in the germinal centers of secondary lymphoid follicles. In other organs, some squamous epithelial and myoepithelial cells were recognized by this antibody. Immunomonitoring of SLCs on light and electron microscopic levels revealed their dendritic morphology, lack of phagosomes, and their close association with type IV collagen fibers. Considering the occurrence of typical dendritic SLCs on the front line of antigen flood, we propose that SLCs be investigated for a possible antigen-binding property.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alles J. U., Bosslet K. Immunohistochemical and immunochemical characterization of a new endothelial cell-specific antigen. J Histochem Cytochem. 1986 Feb;34(2):209–214. doi: 10.1177/34.2.2418100. [DOI] [PubMed] [Google Scholar]
- Amiot M., Bernard A., Raynal B., Knapp W., Deschildre C., Boumsell L. Heterogeneity of the first cluster of differentiation: characterization and epitopic mapping of three CD1 molecules on normal human thymus cells. J Immunol. 1986 Mar 1;136(5):1752–1758. [PubMed] [Google Scholar]
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CASTLEMAN B., IVERSON L., MENENDEZ V. P. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956 Jul-Aug;9(4):822–830. doi: 10.1002/1097-0142(195607/08)9:4<822::aid-cncr2820090430>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Cartun R. W., Coles F. B., Pastuszak W. T. Utilization of monoclonal antibody L26 in the identification and confirmation of B-cell lymphomas. A sensitive and specific marker applicable to formalin-and B5-fixed, paraffin-embedded tissues. Am J Pathol. 1987 Dec;129(3):415–421. [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W. The complement of desmosomal plaque proteins in different cell types. J Cell Biol. 1985 Oct;101(4):1442–1454. doi: 10.1083/jcb.101.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H. G., Gaedicke G., Lok M. S., Diehl V., Minowada J. Hodgkin's disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res. 1986;10(5):487–500. doi: 10.1016/0145-2126(86)90084-6. [DOI] [PubMed] [Google Scholar]
- Feller A. C., Parwaresch M. R., Wacker H. H., Radzun H. J., Lennert K. Combined immunohistochemical staining for surface IgD and T-lymphocyte subsets with monoclonal antibodies in human tonsils. Histochem J. 1983 Jun;15(6):557–562. doi: 10.1007/BF01954146. [DOI] [PubMed] [Google Scholar]
- Feller A. C., Wacker H. H., Moldenhauer G., Radzun H. J., Parwaresch M. R. Monoclonal antibody Ki-B3 detects a formalin resistant antigen on normal and neoplastic B cells. Blood. 1987 Sep;70(3):629–636. [PubMed] [Google Scholar]
- Flavell D. J., Jones D. B., Wright D. H. Identification of tissue histiocytes on paraffin sections by a new monoclonal antibody. J Histochem Cytochem. 1987 Nov;35(11):1217–1226. doi: 10.1177/35.11.3309045. [DOI] [PubMed] [Google Scholar]
- Fujisaku A., Harley J. B., Frank M. B., Gruner B. A., Frazier B., Holers V. M. Genomic organization and polymorphisms of the human C3d/Epstein-Barr virus receptor. J Biol Chem. 1989 Feb 5;264(4):2118–2125. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Grouard G., Durand I., Filgueira L., Banchereau J., Liu Y. J. Dendritic cells capable of stimulating T cells in germinal centres. Nature. 1996 Nov 28;384(6607):364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- HAN S. S. The ultrastructure of the mesenteric lymph node of the rat. Am J Anat. 1961 Sep;109:183–225. doi: 10.1002/aja.1001090208. [DOI] [PubMed] [Google Scholar]
- Haferkamp O., Rosenau W., Lennert K. Vascular transformation of lymph node sinuses due to venous obstruction. Arch Pathol. 1971 Aug;92(2):81–83. [PubMed] [Google Scholar]
- Hansmann M. L., Radzun H. J., Kaiserling E., Parwaresch M. R. Immunoelectron microscopic demonstration of tissue antigens with monoclonal antibodies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;46(1-2):1–12. doi: 10.1007/BF02890290. [DOI] [PubMed] [Google Scholar]
- Hansmann M. L., Wacker H. H., Gralla J., Lumbeck H., Kossmahl M., Heidebrecht H. J., Radzun H. J., Parwaresch M. R. Ki-B5: a monoclonal antibody unrelated to CD45 recognizes normal and neoplastic human B cells in routine paraffin sections. Blood. 1991 Feb 15;77(4):809–817. [PubMed] [Google Scholar]
- Ishii Y., Takami T., Yuasa H., Takei T., Kikuchi K. Two distinct antigen systems in human B lymphocytes: identification of cell surface and intracellular antigens using monoclonal antibodies. Clin Exp Immunol. 1984 Oct;58(1):183–192. [PMC free article] [PubMed] [Google Scholar]
- Kamesaki H., Fukuhara S., Tatsumi E., Uchino H., Yamabe H., Miwa H., Shirakawa S., Hatanaka M., Honjo T. Cytochemical, immunologic, chromosomal, and molecular genetic analysis of a novel cell line derived from Hodgkin's disease. Blood. 1986 Jul;68(1):285–292. [PubMed] [Google Scholar]
- Kan E. A., Wang C. Y., Wang L. C., Evans R. L. Noncovalently bonded subunits of 22 and 28 kd are rapidly internalized by T cells reacted with anti-Leu-4 antibody. J Immunol. 1983 Aug;131(2):536–539. [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Keller A. R., Kaplan H. S., Lukes R. J., Rappaport H. Correlation of histopathology with other prognostic indicators in Hodgkin's disease. Cancer. 1968 Sep;22(3):487–499. doi: 10.1002/1097-0142(196809)22:3<487::aid-cncr2820220302>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Xu J., de Bouteiller O., Parham C. L., Grouard G., Djossou O., de Saint-Vis B., Lebecque S., Banchereau J., Moore K. W. Follicular dendritic cells specifically express the long CR2/CD21 isoform. J Exp Med. 1997 Jan 6;185(1):165–170. doi: 10.1084/jem.185.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. D., Cooper N. R., Tack B. F., Nemerow G. R. Molecular cloning of the cDNA encoding the Epstein-Barr virus/C3d receptor (complement receptor type 2) of human B lymphocytes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9194–9198. doi: 10.1073/pnas.84.24.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Feller A. C., Peters K. P., Hansmann M. L. Peroxidase-positive mononuclear leukocytes as possible precursors of human dendritic reticulum cells. J Immunol. 1983 Dec;131(6):2719–2725. [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Hansmann M. L., Peters K. P. Monoclonal antibody Ki-M4 specifically recognizes human dendritic reticulum cells (follicular dendritic cells) and their possible precursor in blood. Blood. 1983 Sep;62(3):585–590. [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Kreipe H., Hansmann M. L., Barth J. Monocyte/macrophage-reactive monoclonal antibody Ki-M6 recognizes an intracytoplasmic antigen. Am J Pathol. 1986 Oct;125(1):141–151. [PMC free article] [PubMed] [Google Scholar]
- Peters J., Krams M., Wacker H. H., Carstens A., Weisner D., Hamann K., Menke M., Harms D., Parwaresch R. Detection of rare RNA sequences by single-enzyme in situ reverse transcription-polymerase chain reaction. High-resolution analyses of interleukin-6 mRNA in paraffin sections of lymph nodes. Am J Pathol. 1997 Feb;150(2):469–476. [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford K. A., Rigney E. M., Micklem K. J., Jones M., Stross W. P., Gatter K. C., Mason D. Y. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989 Apr;42(4):414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzun H. J., Hansmann M. L., Heidebrecht H. J., Bödewadt-Radzun S., Wacker H. H., Kreipe H., Lumbeck H., Hernandez C., Kuhn C., Parwaresch M. R. Detection of a monocyte/macrophage differentiation antigen in routinely processed paraffin-embedded tissues by monoclonal antibody Ki-M1P. Lab Invest. 1991 Sep;65(3):306–315. [PubMed] [Google Scholar]
- Radzun H. J., Kreipe H., Bödewadt S., Hansmann M. L., Barth J., Parwaresch M. R. Ki-M8 monoclonal antibody reactive with an intracytoplasmic antigen of monocyte/macrophage lineage. Blood. 1987 May;69(5):1320–1327. [PubMed] [Google Scholar]
- Radzun H. J., Parwaresch M. R., Feller A. C., Hansmann M. L. Monocyte/macrophage-specific monoclonal antibody Ki-M1 recognizes interdigitating reticulum cells. Am J Pathol. 1984 Dec;117(3):441–450. [PMC free article] [PubMed] [Google Scholar]
- Robb-Smith A. H. U.S. National Cancer Institute working formulation of non-Hodgkin's lymphomas for clinical use. Lancet. 1982 Aug 21;2(8295):432–434. doi: 10.1016/s0140-6736(82)90454-8. [DOI] [PubMed] [Google Scholar]
- Saalbach A., Aneregg U., Bruns M., Schnabel E., Herrmann K., Haustein U. F. Novel fibroblast-specific monoclonal antibodies: properties and specificities. J Invest Dermatol. 1996 Jun;106(6):1314–1319. doi: 10.1111/1523-1747.ep12349035. [DOI] [PubMed] [Google Scholar]
- Schaadt M., Diehl V., Stein H., Fonatsch C., Kirchner H. H. Two neoplastic cell lines with unique features derived from Hodgkin's disease. Int J Cancer. 1980 Dec 15;26(6):723–731. doi: 10.1002/ijc.2910260605. [DOI] [PubMed] [Google Scholar]
- Stansfeld A. G., Diebold J., Noel H., Kapanci Y., Rilke F., Kelényi G., Sundstrom C., Lennert K., van Unnik J. A., Mioduszewska O. Updated Kiel classification for lymphomas. Lancet. 1988 Feb 6;1(8580):292–293. doi: 10.1016/s0140-6736(88)90367-4. [DOI] [PubMed] [Google Scholar]
- Stein H., Bonk A., Tolksdorf G., Lennert K., Rodt H., Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980 Aug;28(8):746–760. doi: 10.1177/28.8.7003001. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Szakal A. K., Holmes K. L., Tew J. G. Transport of immune complexes from the subcapsular sinus to lymph node follicles on the surface of nonphagocytic cells, including cells with dendritic morphology. J Immunol. 1983 Oct;131(4):1714–1727. [PubMed] [Google Scholar]
- Tabrizchi H., Hansmann M. L., Parwaresch M. R., Lennert K. Distribution pattern of follicular dendritic cells in low grade B-cell lymphomas of the gastrointestinal tract immunostained by Ki-FDC1p: a new paraffin-resistant monoclonal antibody. Mod Pathol. 1990 Jul;3(4):470–478. [PubMed] [Google Scholar]
- Takahashi K., Isobe T., Ohtsuki Y., Sonobe H., Takeda I., Akagi T. Immunohistochemical localization and distribution of S-100 proteins in the human lymphoreticular system. Am J Pathol. 1984 Sep;116(3):497–503. [PMC free article] [PubMed] [Google Scholar]
- Tew J. G., Thorbecke G. J., Steinman R. M. Dendritic cells in the immune response: characteristics and recommended nomenclature (A report from the Reticuloendothelial Society Committee on Nomenclature). J Reticuloendothel Soc. 1982 May;31(5):371–380. [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Ugolini V., Nunez G., Smith R. G., Stastny P., Capra J. D. Initial characterization of monoclonal antibodies against human monocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6764–6768. doi: 10.1073/pnas.77.11.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J. J., Toothaker L. E., Smith J. A., Weis J. H., Fearon D. T. Structure of the human B lymphocyte receptor for C3d and the Epstein-Barr virus and relatedness to other members of the family of C3/C4 binding proteins. J Exp Med. 1988 Mar 1;167(3):1047–1066. doi: 10.1084/jem.167.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Valk P., Meijer C. J. The histology of reactive lymph nodes. Am J Surg Pathol. 1987 Nov;11(11):866–882. doi: 10.1097/00000478-198711000-00005. [DOI] [PubMed] [Google Scholar]