Abstract
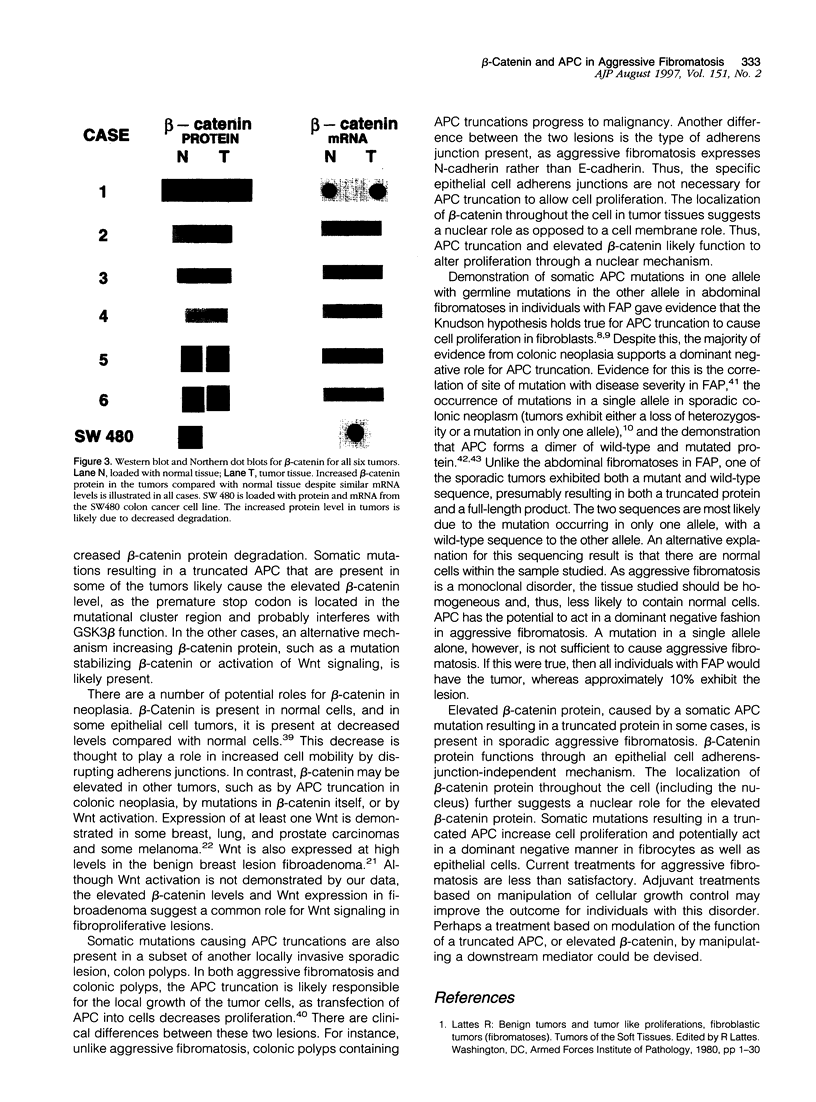
Sporadic aggressive fibromatosis (also called desmoid tumor) is a monoclonal proliferation of spindle (fibrocyte-like) cells that is locally invasive but does not metastasize. A similarity to abdominal fibromatoses (desmoids) in familial adenomatous polyposis and a cytogenetic study showing partial deletion of 5q in a subset of aggressive fibromatoses suggests that the adenomatous polyposis coli (APC) gene plays a role in its pathogenesis. APC helps regulate the cellular level of beta-catenin, which is a downstream mediator in Wnt (Wingless) signaling. beta-Catenin has a nuclear function (binds transcription factors) and a cell membrane function (is a component of epithelial cell adherens junctions). Six cases of aggressive fibromatosis of the extremities from patients without familial adenomatous polyposis, or a family history of colon cancer, were studied. Immunohistochemistry, using carboxy and amino terminus antibodies to APC, and DNA sequencing showed that three of the six contained an APC-truncating mutation, whereas normal tissues did not contain a mutation. Western blot and Northern dot blot showed that all six tumors had a higher level of beta-catenin protein than surrounding normal tissues, despite containing similar levels of beta-catenin mRNA. Immunohistochemistry localized beta-catenin throughout the cell in tumor tissues, although it localized more to the periphery in cells from normal tissues. Reverse transcription polymerase chain reaction showed that the tumors expressed N-cadherin but not E-cadherin (a pattern of expression of proteins making up adherens junctions similar to fibrocytes), suggesting that the specific adherens junctions present in epithelial cells are not necessary for beta-catenin function. Increased beta-catenin may cause the growth advantage of cells in this tumor through a nuclear mechanism. The increased protein level, relative to the RNA level, suggests that beta-catenin is degraded at a lower rate compared with normal tissues. In some cases, this is caused by a somatic mutation resulting in a truncated APC protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alman B. A., Goldberg M. J., Naber S. P., Galanopoulous T., Antoniades H. N., Wolfe H. J. Aggressive fibromatosis. J Pediatr Orthop. 1992 Jan;12(1):1–10. [PubMed] [Google Scholar]
- Alman B. A., Greel D. A., Ruby L. K., Goldberg M. J., Wolfe H. J. Regulation of proliferation and platelet-derived growth factor expression in palmar fibromatosis (Dupuytren contracture) by mechanical strain. J Orthop Res. 1996 Sep;14(5):722–728. doi: 10.1002/jor.1100140507. [DOI] [PubMed] [Google Scholar]
- Alman B. A., Pajerski M. E., Diaz-Cano S., Corboy K., Wolfe H. J. Aggressive fibromatosis (desmoid tumor) is a monoclonal disorder. Diagn Mol Pathol. 1997 Apr;6(2):98–101. doi: 10.1097/00019606-199704000-00005. [DOI] [PubMed] [Google Scholar]
- Armeanu S., Bühring H. J., Reuss-Borst M., Müller C. A., Klein G. E-cadherin is functionally involved in the maturation of the erythroid lineage. J Cell Biol. 1995 Oct;131(1):243–249. doi: 10.1083/jcb.131.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg G. H., Matsumine A., Kuroda T., Bhattacharjee R. N., Miyashiro I., Toyoshima K., Akiyama T. The tumour suppressor gene product APC blocks cell cycle progression from G0/G1 to S phase. EMBO J. 1995 Nov 15;14(22):5618–5625. doi: 10.1002/j.1460-2075.1995.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J. A., Sreekantaiah C., Mouron B., Neff J. R., Sandberg A. A., Wolman S. R. Clonal chromosomal abnormalities in desmoid tumors. Implications for histopathogenesis. Cancer. 1992 Jan 15;69(2):430–436. doi: 10.1002/1097-0142(19920115)69:2<430::aid-cncr2820690226>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Doki Y., Shiozaki H., Tahara H., Inoue M., Oka H., Iihara K., Kadowaki T., Takeichi M., Mori T. Correlation between E-cadherin expression and invasiveness in vitro in a human esophageal cancer cell line. Cancer Res. 1993 Jul 15;53(14):3421–3426. [PubMed] [Google Scholar]
- Evans K., al-Maghtheh M., Fitzke F. W., Moore A. T., Jay M., Inglehearn C. F., Arden G. B., Bird A. C. Bimodal expressivity in dominant retinitis pigmentosa genetically linked to chromosome 19q. Br J Ophthalmol. 1995 Sep;79(9):841–846. doi: 10.1136/bjo.79.9.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Guger K. A., Gumbiner B. M. beta-Catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev Biol. 1995 Nov;172(1):115–125. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995 Oct;7(5):634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Gurbuz A. K., Giardiello F. M., Petersen G. M., Krush A. J., Offerhaus G. J., Booker S. V., Kerr M. C., Hamilton S. R. Desmoid tumours in familial adenomatous polyposis. Gut. 1994 Mar;35(3):377–381. doi: 10.1136/gut.35.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Ichii S., Nagase H., Nakamura Y. Multiple forms of the APC gene transcripts and their tissue-specific expression. Hum Mol Genet. 1993 Mar;2(3):283–287. doi: 10.1093/hmg/2.3.283. [DOI] [PubMed] [Google Scholar]
- Huguet E. L., McMahon J. A., McMahon A. P., Bicknell R., Harris A. L. Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Cancer Res. 1994 May 15;54(10):2615–2621. [PubMed] [Google Scholar]
- Hülsken J., Birchmeier W., Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol. 1994 Dec;127(6 Pt 2):2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara A., Koizumi H., Hashizume R., Uchikoshi T. Expression of epithelial cadherin and alpha- and beta-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology. 1996 Jun;23(6):1441–1447. doi: 10.1053/jhep.1996.v23.pm0008675162. [DOI] [PubMed] [Google Scholar]
- Inomata M., Ochiai A., Akimoto S., Kitano S., Hirohashi S. Alteration of beta-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res. 1996 May 1;56(9):2213–2217. [PubMed] [Google Scholar]
- Iozzo R. V., Eichstetter I., Danielson K. G. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995 Aug 15;55(16):3495–3499. [PubMed] [Google Scholar]
- Joslyn G., Richardson D. S., White R., Alber T. Dimer formation by an N-terminal coiled coil in the APC protein. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11109–11113. doi: 10.1073/pnas.90.23.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C., Peifer M. Not just glue: cell-cell junctions as cellular signaling centers. Curr Opin Genet Dev. 1995 Feb;5(1):56–65. doi: 10.1016/s0959-437x(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997 Mar 21;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Li M., Cordon-Cardo C., Gerald W. L., Rosai J. Desmoid fibromatosis is a clonal process. Hum Pathol. 1996 Sep;27(9):939–943. doi: 10.1016/s0046-8177(96)90221-x. [DOI] [PubMed] [Google Scholar]
- Miyaki M., Konishi M., Kikuchi-Yanoshita R., Enomoto M., Igari T., Tanaka K., Muraoka M., Takahashi H., Amada Y., Fukayama M. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994 Jun 1;54(11):3011–3020. [PubMed] [Google Scholar]
- Miyaki M., Konishi M., Kikuchi-Yanoshita R., Enomoto M., Tanaka K., Takahashi H., Muraoka M., Mori T., Konishi F., Iwama T. Coexistence of somatic and germ-line mutations of APC gene in desmoid tumors from patients with familial adenomatous polyposis. Cancer Res. 1993 Nov 1;53(21):5079–5082. [PubMed] [Google Scholar]
- Miyoshi Y., Nagase H., Ando H., Horii A., Ichii S., Nakatsuru S., Aoki T., Miki Y., Mori T., Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992 Jul;1(4):229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997 Mar 21;275(5307):1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Miyoshi Y., Horii A., Aoki T., Ogawa M., Utsunomiya J., Baba S., Sasazuki T., Nakamura Y. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res. 1992 Jul 15;52(14):4055–4057. [PubMed] [Google Scholar]
- Papkoff J., Rubinfeld B., Schryver B., Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol. 1996 May;16(5):2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. C., Shiu M. H., Newsome J. L., Hajdu S. I., Gaynor J. J., Brennan M. F. The desmoid tumor. Not a benign disease. Arch Surg. 1989 Feb;124(2):191–196. doi: 10.1001/archsurg.1989.01410020061010. [DOI] [PubMed] [Google Scholar]
- Ringwald M., Schuh R., Vestweber D., Eistetter H., Lottspeich F., Engel J., Dölz R., Jähnig F., Epplen J., Mayer S. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B., Robbins P., El-Gamil M., Albert I., Porfiri E., Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997 Mar 21;275(5307):1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Schulze C., Firth J. A. Immunohistochemical localization of adherens junction components in blood-brain barrier microvessels of the rat. J Cell Sci. 1993 Mar;104(Pt 3):773–782. doi: 10.1242/jcs.104.3.773. [DOI] [PubMed] [Google Scholar]
- Sen-Gupta S., Van der Luijt R. B., Bowles L. V., Meera Khan P., Delhanty J. D. Somatic mutation of APC gene in desmoid tumour in familial adenomatous polyposis. Lancet. 1993 Aug 28;342(8870):552–553. doi: 10.1016/0140-6736(93)91677-e. [DOI] [PubMed] [Google Scholar]
- Siegfried E., Perrimon N. Drosophila wingless: a paradigm for the function and mechanism of Wnt signaling. Bioessays. 1994 Jun;16(6):395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- Su L. K., Johnson K. A., Smith K. J., Hill D. E., Vogelstein B., Kinzler K. W. Association between wild type and mutant APC gene products. Cancer Res. 1993 Jun 15;53(12):2728–2731. [PubMed] [Google Scholar]
- Takayama T., Shiozaki H., Shibamoto S., Oka H., Kimura Y., Tamura S., Inoue M., Monden T., Ito F., Monden M. Beta-catenin expression in human cancers. Am J Pathol. 1996 Jan;148(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- Yonemura S., Itoh M., Nagafuchi A., Tsukita S. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci. 1995 Jan;108(Pt 1):127–142. doi: 10.1242/jcs.108.1.127. [DOI] [PubMed] [Google Scholar]