Abstract
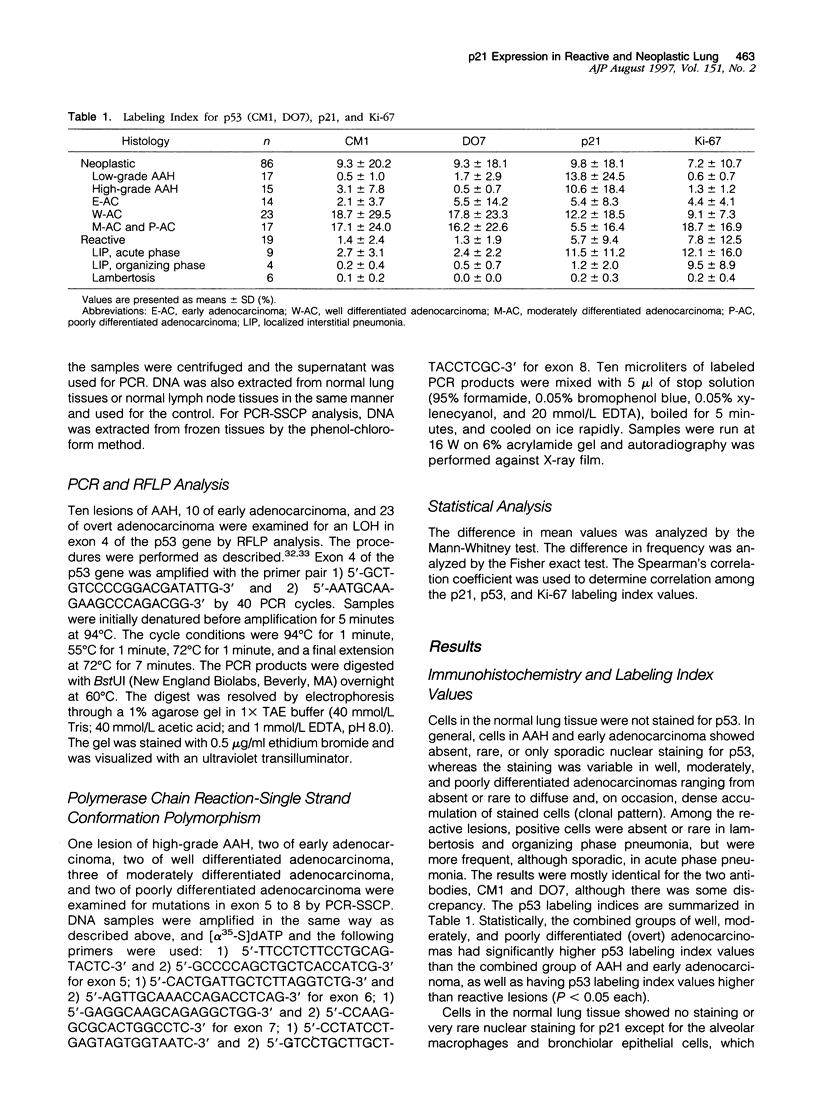
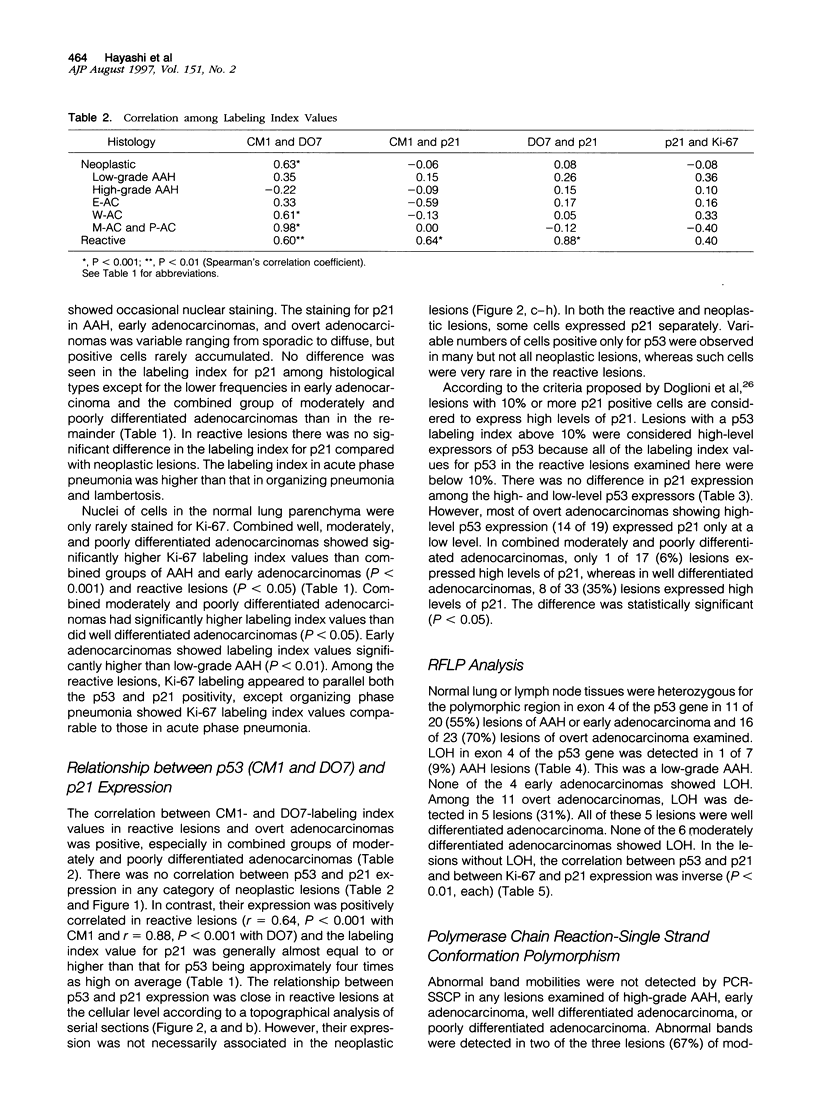
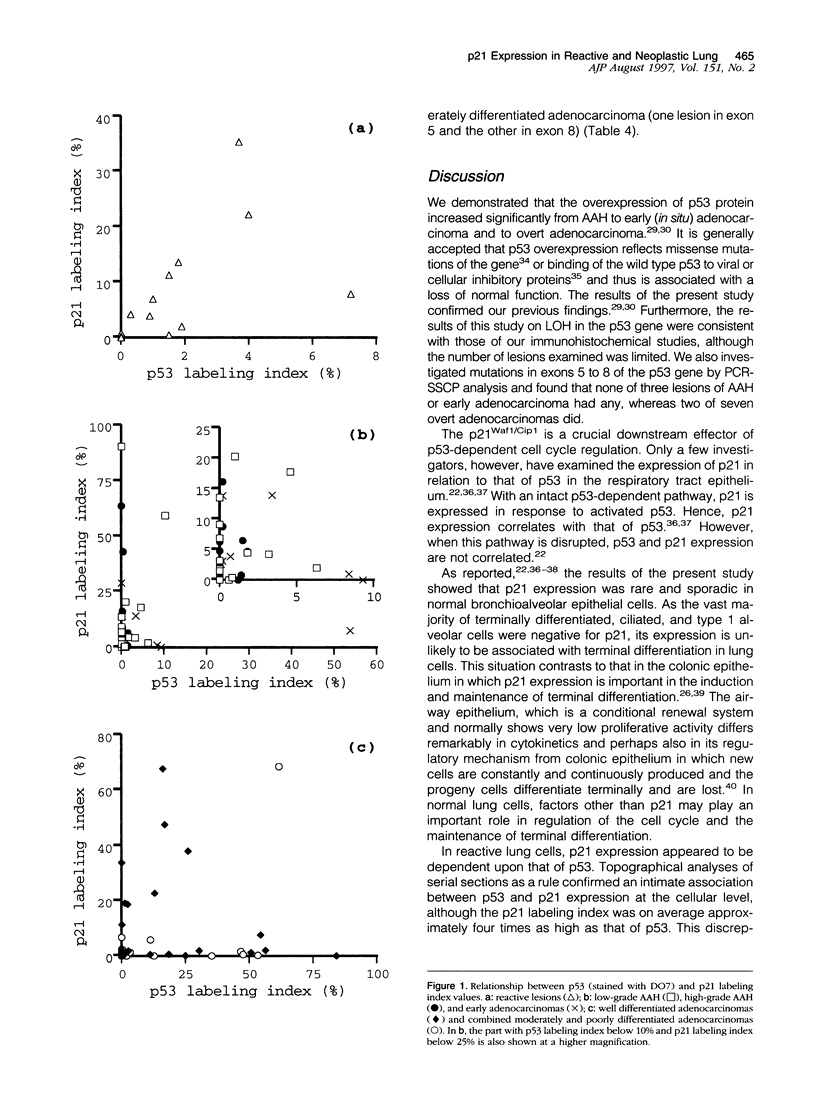
Our studies suggested that adenocarcinoma of the peripheral lung mostly develops by several steps from atypical adenomatous hyperplasia through early adenocarcinoma to overt adenocarcinoma, and that some p53 abnormalities play an important role in this progression. In the present study, we examined by immunohistochemistry the expression of p53-inducible cyclin-dependent kinase inhibitor p21Waf1/Cip1 (p21) in the cells at various developmental stages of lung adenocarcinoma (32 lesions of adenomatous hyperplasia, 14 of early adenocarcinoma, 23 of well differentiated adenocarcinoma, and 17 of moderately or poorly differentiated adenocarcinoma) in comparison with 19 reactive proliferative lesions and analyzed the relationship between p53 and p21 expression. Bronchioalveolar cells in the normal lung expressed very little or no p21 and no p53 expression. In not only reactive but also neoplastic lesions regardless of their developmental stage, the cells expressed p21 at various frequencies. The average labeling indices ranged from 5.4 to 13.8%, and there was no significant difference between any of these categories. The expression of p21, however, tended to be relatively low in moderately and poorly differentiated adenocarcinomas (5.5%) compared to well differentiated adenocarcinomas (12.2%), and high-level p21 expressors (10% < or = positive cells) were more frequent in the latter group (1 of 17 (6%) versus 3 of 23 (35%), P < 0.05), suggesting that p21 expression is affected by the degree of differentiation of the neoplastic cells. Although the correlation was positive between the expression of p21 and p53 in reactive lesions (r = 0.88; P < 0.001), none was found in neoplastic lesions at any step or grade (-0.12 < or = r < or = 0.26). These results indicated that p21 expression depends upon p53 expression in reactive lung cells, whereas p21 expression is at least in part independent of that of p53 from the earliest to the most fully developed step of lung adenocarcinoma tumorigenesis. We concluded that disruption of the p53-dependent cell cycle regulation is a very early event in the tumorigenesis of lung adenocarcinoma.
Full text
PDF

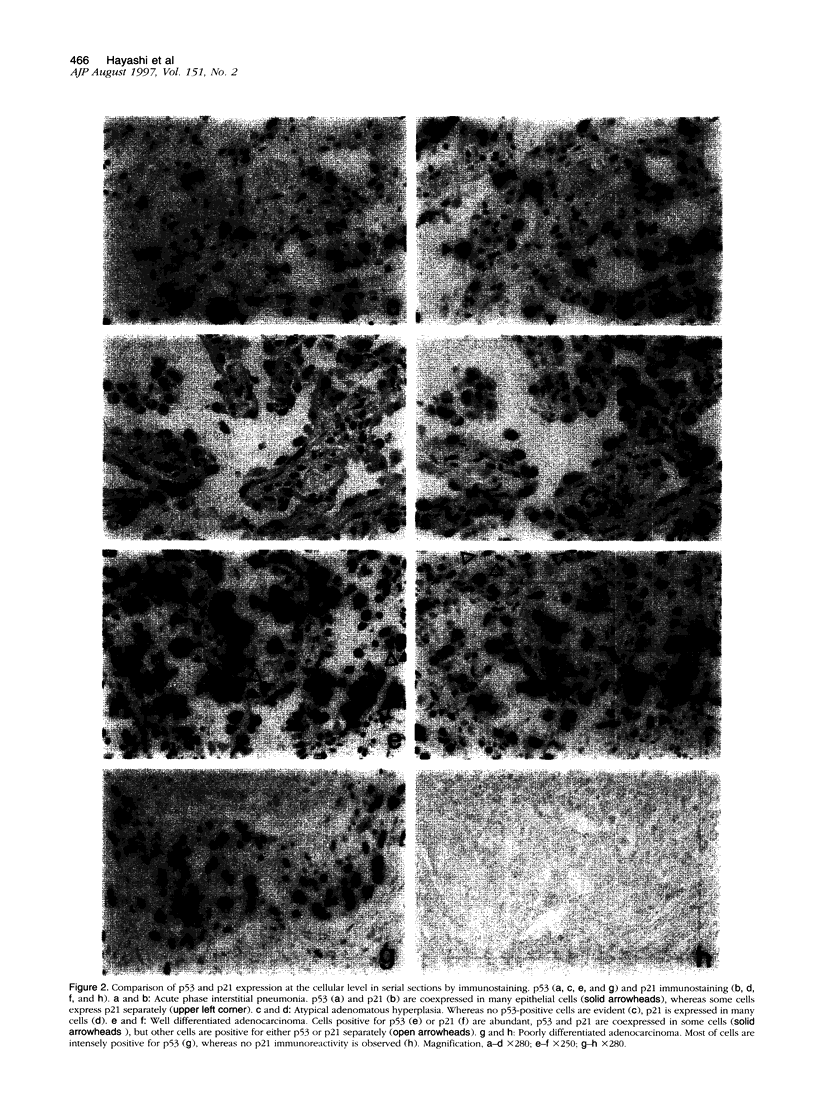
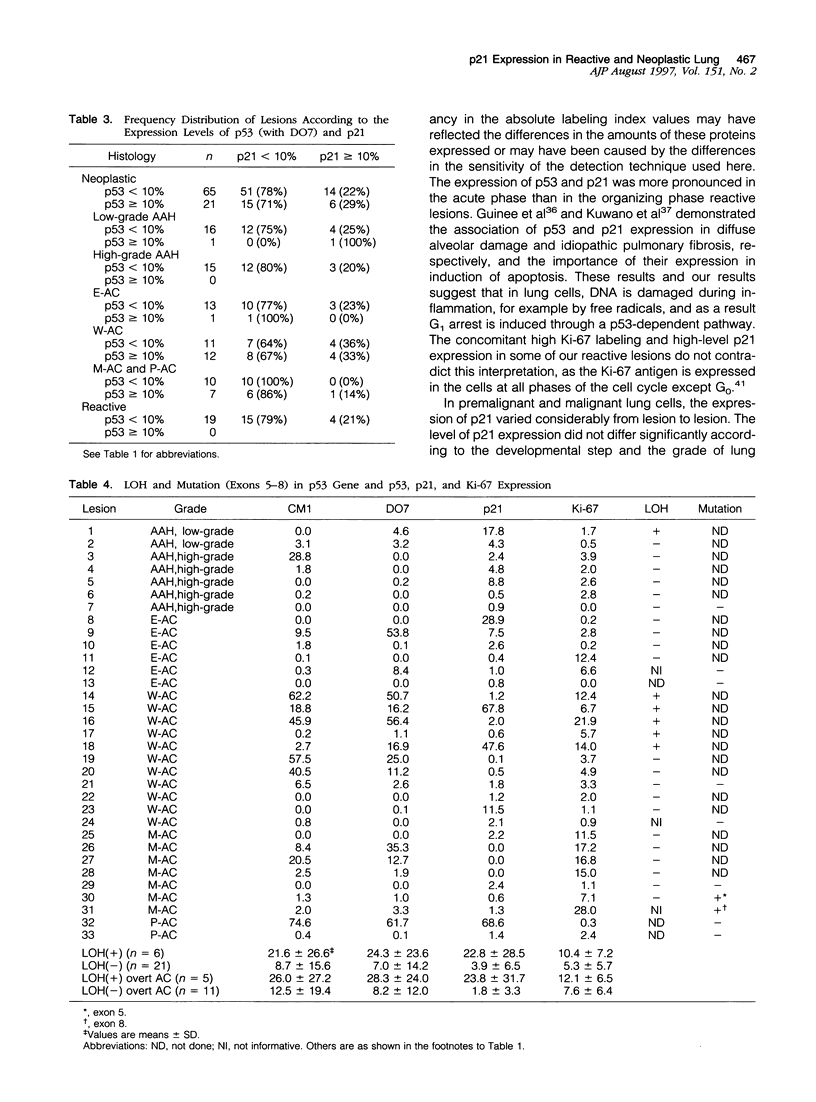
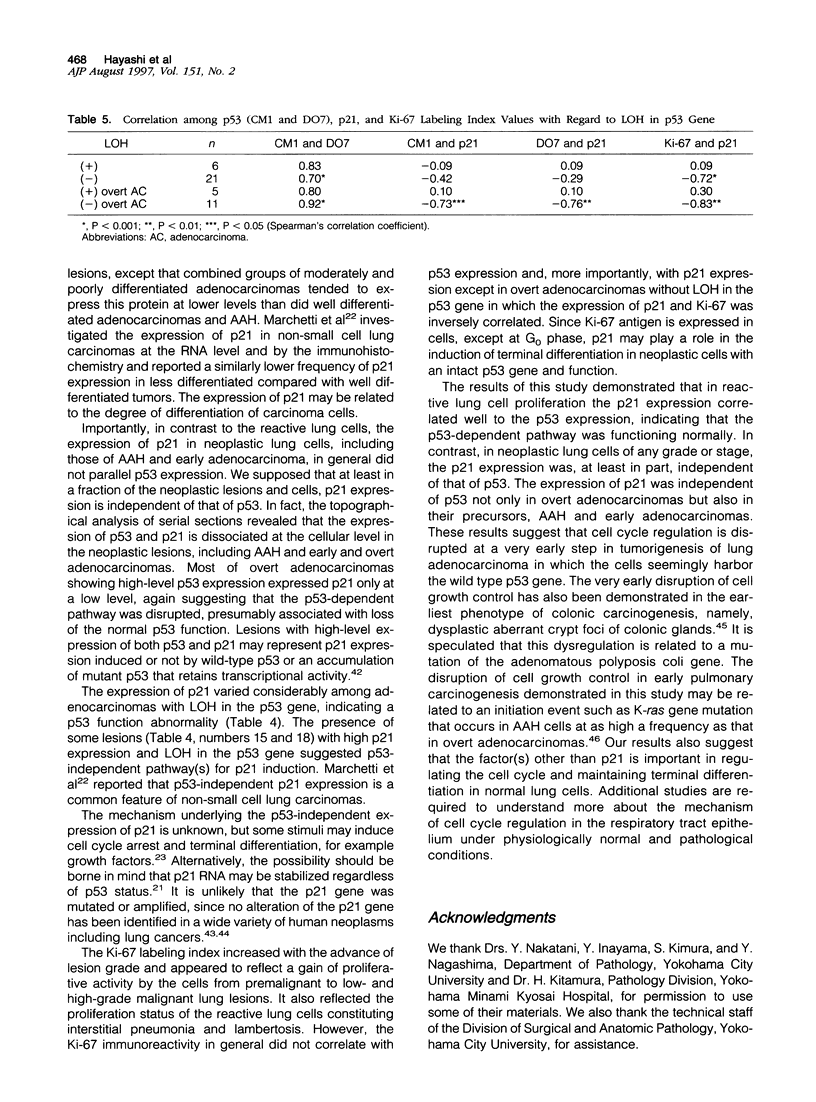


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodner S. M., Minna J. D., Jensen S. M., D'Amico D., Carbone D., Mitsudomi T., Fedorko J., Buchhagen D. L., Nau M. M., Gazdar A. F. Expression of mutant p53 proteins in lung cancer correlates with the class of p53 gene mutation. Oncogene. 1992 Apr;7(4):743–749. [PubMed] [Google Scholar]
- Chiba I., Takahashi T., Nau M. M., D'Amico D., Curiel D. T., Mitsudomi T., Buchhagen D. L., Carbone D., Piantadosi S., Koga H. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990 Oct;5(10):1603–1610. [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Datto M. B., Li Y., Panus J. F., Howe D. J., Xiong Y., Wang X. F. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Redston M. S., Yeo C. J., Kern S. E., Hruban R. H. p53-independent expression of the cyclin-dependent kinase inhibitor p21 in pancreatic carcinoma. Am J Pathol. 1995 Oct;147(4):884–888. [PMC free article] [PubMed] [Google Scholar]
- Doglioni C., Pelosio P., Laurino L., Macri E., Meggiolaro E., Favretti F., Barbareschi M. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. J Pathol. 1996 Jul;179(3):248–253. doi: 10.1002/(SICI)1096-9896(199607)179:3<248::AID-PATH571>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Dosaka-Akita H., Shindoh M., Fujino M., Kinoshita I., Akie K., Katoh M., Kawakami Y. Abnormal p53 expression in human lung cancer is associated with histologic subtypes and patient smoking history. Am J Clin Pathol. 1994 Nov;102(5):660–664. doi: 10.1093/ajcp/102.5.660. [DOI] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fredersdorf S., Milne A. W., Hall P. A., Lu X. Characterization of a panel of novel anti-p21Waf1/Cip1 monoclonal antibodies and immunochemical analysis of p21Waf1/Cip1 expression in normal human tissues. Am J Pathol. 1996 Mar;148(3):825–835. [PMC free article] [PubMed] [Google Scholar]
- Fuchs B., O'Connor D., Fallis L., Scheidtmann K. H., Lu X. p53 phosphorylation mutants retain transcription activity. Oncogene. 1995 Feb 16;10(4):789–793. [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Guinee D., Jr, Fleming M., Hayashi T., Woodward M., Zhang J., Walls J., Koss M., Ferrans V., Travis W. Association of p53 and WAF1 expression with apoptosis in diffuse alveolar damage. Am J Pathol. 1996 Aug;149(2):531–538. [PMC free article] [PubMed] [Google Scholar]
- Hainaut P., Soussi T., Shomer B., Hollstein M., Greenblatt M., Hovig E., Harris C. C., Montesano R. Database of p53 gene somatic mutations in human tumors and cell lines: updated compilation and future prospects. Nucleic Acids Res. 1997 Jan 1;25(1):151–157. doi: 10.1093/nar/25.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Novitch B. G., Spicer D. B., Skapek S. X., Rhee J., Hannon G. J., Beach D., Lassar A. B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995 Feb 17;267(5200):1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Elledge S. J., Keyomarsi K., Dynlacht B., Tsai L. H., Zhang P., Dobrowolski S., Bai C., Connell-Crowley L., Swindell E. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995 Apr;6(4):387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kitamura H., Kameda Y., Nakamura N., Inayama Y., Nakatani Y., Shibagaki T., Ito T., Hayashi H., Kimura H., Kanisawa M. Atypical adenomatous hyperplasia and bronchoalveolar lung carcinoma. Analysis by morphometry and the expressions of p53 and carcinoembryonic antigen. Am J Surg Pathol. 1996 May;20(5):553–562. doi: 10.1097/00000478-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Kitamura H., Kameda Y., Nakamura N., Nakatani Y., Inayama Y., Iida M., Noda K., Ogawa N., Shibagaki T., Kanisawa M. Proliferative potential and p53 overexpression in precursor and early stage lesions of bronchioloalveolar lung carcinoma. Am J Pathol. 1995 Apr;146(4):876–887. [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano K., Kunitake R., Kawasaki M., Nomoto Y., Hagimoto N., Nakanishi Y., Hara N. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996 Aug;154(2 Pt 1):477–483. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- Li Y. J., Laurent-Puig P., Salmon R. J., Thomas G., Hamelin R. Polymorphisms and probable lack of mutation in the WAF1-CIP1 gene in colorectal cancer. Oncogene. 1995 Feb 2;10(3):599–601. [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Macleod K. F., Sherry N., Hannon G., Beach D., Tokino T., Kinzler K., Vogelstein B., Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995 Apr 15;9(8):935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- Marchetti A., Doglioni C., Barbareschi M., Buttitta F., Pellegrini S., Bertacca G., Chella A., Merlo G., Angeletti C. A., Dalla Palma P. p21 RNA and protein expression in non-small cell lung carcinomas: evidence of p53-independent expression and association with tumoral differentiation. Oncogene. 1996 Mar 21;12(6):1319–1324. [PubMed] [Google Scholar]
- Meltzer S. J., Yin J., Huang Y., McDaniel T. K., Newkirk C., Iseri O., Vogelstein B., Resau J. H. Reduction to homozygosity involving p53 in esophageal cancers demonstrated by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4976–4980. doi: 10.1073/pnas.88.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Miyamoto H., Kubota Y., Shuin T., Torigoe S., Hosaka M., Iwasaki Y., Danenberg K., Danenberg P. V. Analyses of p53 gene mutations in primary human bladder cancer. Oncol Res. 1993;5(6-7):245–249. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Polyak K., Hamilton S. R., Vogelstein B., Kinzler K. W. Early alteration of cell-cycle-regulated gene expression in colorectal neoplasia. Am J Pathol. 1996 Aug;149(2):381–387. [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Sato T., Kurose A., Ikeda E. Immunohistochemical detection of p21waf1/cip1/sdi1 and p53 proteins in formalin-fixed, paraffin-embedded tissue sections of colorectal carcinoma. Hum Pathol. 1996 Sep;27(9):912–916. doi: 10.1016/s0046-8177(96)90217-8. [DOI] [PubMed] [Google Scholar]
- Schwaller J., Koeffler H. P., Niklaus G., Loetscher P., Nagel S., Fey M. F., Tobler A. Posttranscriptional stabilization underlies p53-independent induction of p21WAF1/CIP1/SDI1 in differentiating human leukemic cells. J Clin Invest. 1995 Mar;95(3):973–979. doi: 10.1172/JCI117806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohara M., el-Deiry W. S., Wada M., Nakamaki T., Takeuchi S., Yang R., Chen D. L., Vogelstein B., Koeffler H. P. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994 Dec 1;84(11):3781–3784. [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Westra W. H., Baas I. O., Hruban R. H., Askin F. B., Wilson K., Offerhaus G. J., Slebos R. J. K-ras oncogene activation in atypical alveolar hyperplasias of the human lung. Cancer Res. 1996 May 1;56(9):2224–2228. [PubMed] [Google Scholar]
- Wooding F. B., Morgan G., Jones G. V., Care A. D. Calcium transport and the localisation of calbindin-D9k in the ruminant placenta during the second half of pregnancy. Cell Tissue Res. 1996 Sep;285(3):477–489. doi: 10.1007/s004410050664. [DOI] [PubMed] [Google Scholar]
- Yasui W., Akama Y., Kuniyasu H., Yokozaki H., Semba S., Shimamoto F., Tahara E. Expression of cyclin-dependent kinase inhibitor p21WAF1/CIP1 in non-neoplastic mucosa and neoplasia of the stomach: relationship with p53 status and proliferative activity. J Pathol. 1996 Oct;180(2):122–128. doi: 10.1002/(SICI)1096-9896(199610)180:2<122::AID-PATH647>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zhang W., Grasso L., McClain C. D., Gambel A. M., Cha Y., Travali S., Deisseroth A. B., Mercer W. E. p53-independent induction of WAF1/CIP1 in human leukemia cells is correlated with growth arrest accompanying monocyte/macrophage differentiation. Cancer Res. 1995 Feb 1;55(3):668–674. [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]