Abstract
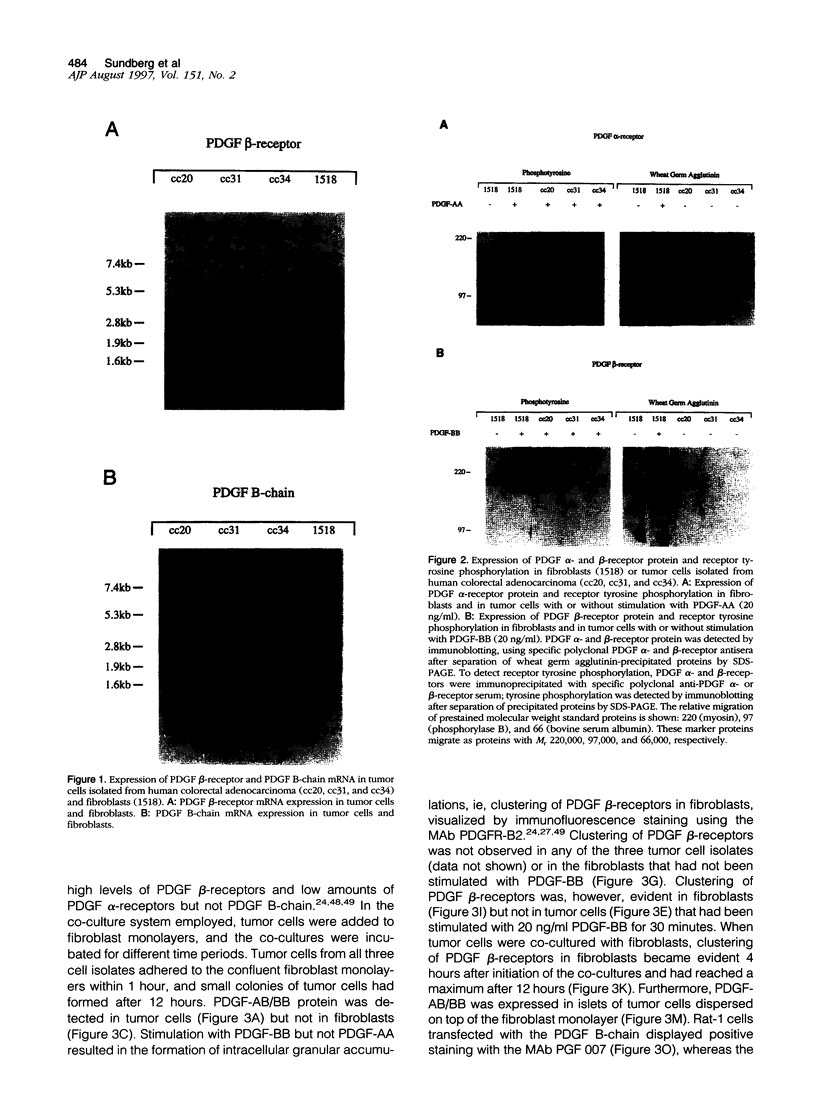
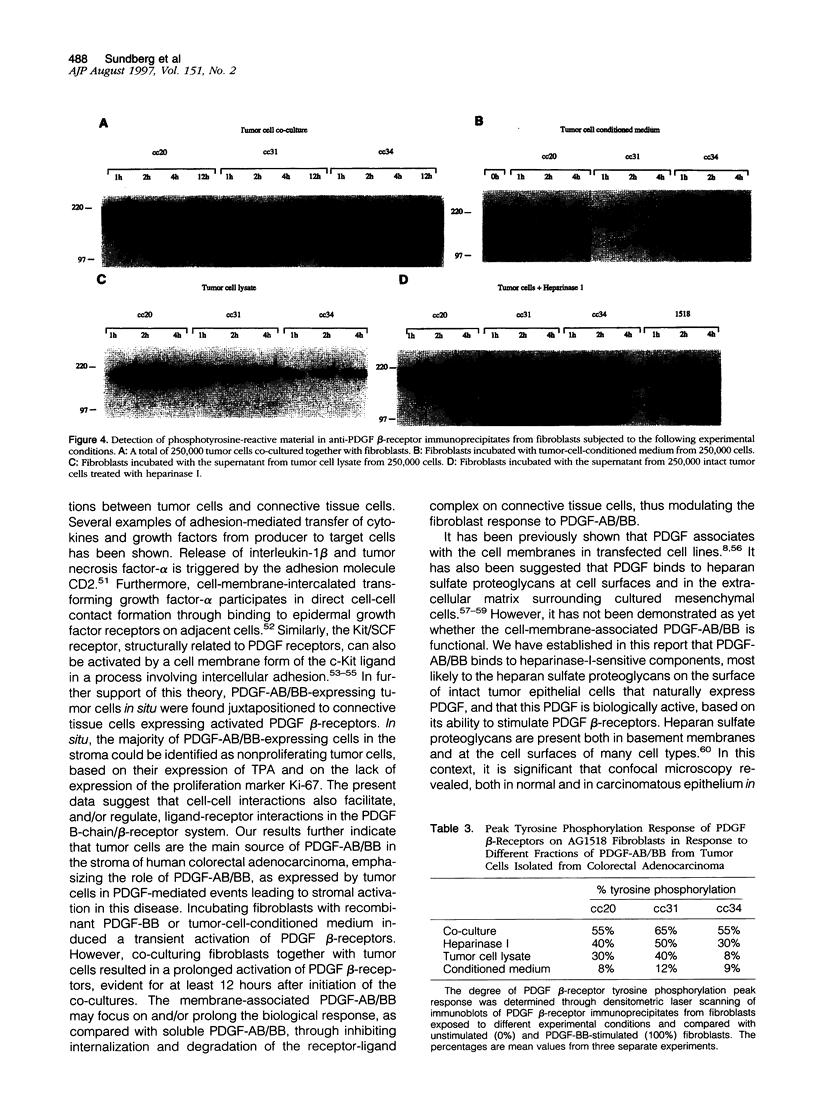
Mechanisms underlying stimulation of platelet-derived growth factor (PDGF) beta-receptors expressed on connective tissue cells in human colorectal adenocarcinoma were investigated in this study. PDGF-AB/BB, but not PDGF receptors, was expressed by tumor cells in situ, as well as in tumor cell isolates of low passage from human colorectal adenocarcinoma. In an experimental co-culture system, conditioned medium from tumor cells only marginally activated PDGF beta-receptors expressed on fibroblasts. In contrast, co-culturing of the two cell types led to a marked PDGF beta-receptor activation. Functional PDGF-AB/BB was found to be associated with heparinase-I-sensitive components on the tumor cell surface. PDGF-AB/BB, isolated from heparinase-I-sensitive cell surface components, induced a marked activation of PDGF beta-receptors. Furthermore, co-culturing tumor cells together with fibroblasts led to a sustained activation of PDGF beta-receptors expressed on fibroblasts. Double immunofluorescence staining of tissue sections from human colorectal adenocarcinoma, combined with computer-aided image analysis, revealed that nonproliferating tumor cells were the predominant cellular source of PDGF-AB/BB in the tumor stroma. In addition, PDGF-AB/BB-expressing tumor cells were found juxtapositioned to microvascular cells expressing activated PDGF beta-receptors. Confocal microscopy revealed a cytoplasmic and cell-membrane-associated expression of PDGF-AB/BB in tumor cells situated in the stroma. In contrast, epithelial cells situated in normal or tumorous acinar structures revealed only a cell-membrane-associated PDGF-AB/BB expression. The is vitro and in situ results demonstrate that tumor cells not only facilitate but also have the ability to modulate connective tissue cell responsiveness to PDGF-AB/BB in a paracrine fashion, through direct cell-cell interactions in human colorectal adenocarcinoma.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi S., Ebi Y., Nishikawa S., Hayashi S., Yamazaki M., Kasugai T., Yamamura T., Nomura S., Kitamura Y. Necessity of extracellular domain of W (c-kit) receptors for attachment of murine cultured mast cells to fibroblasts. Blood. 1992 Feb 1;79(3):650–656. [PubMed] [Google Scholar]
- Anklesaria P., Teixidó J., Laiho M., Pierce J. H., Greenberger J. S., Massagué J. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc Natl Acad Sci U S A. 1990 May;87(9):3289–3293. doi: 10.1073/pnas.87.9.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzano M. A., Rieman D., Prichett W., Bowen-Pope D. F., Greig R. Growth factor production by human colon carcinoma cell lines. Cancer Res. 1989 Jun 1;49(11):2898–2904. [PubMed] [Google Scholar]
- Avraham H., Scadden D. T., Chi S., Broudy V. C., Zsebo K. M., Groopman J. E. Interaction of human bone marrow fibroblasts with megakaryocytes: role of the c-kit ligand. Blood. 1992 Oct 1;80(7):1679–1684. [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman F. T., de Bruïne A., Flohil C., van der Wurff A., ten Kate J., Dinjens W. W. Epithelial-stromal interactions in colon cancer. Int J Dev Biol. 1993 Mar;37(1):203–211. [PubMed] [Google Scholar]
- Burger P. C., Shibata T., Kleihues P. The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: application to surgical neuropathology. Am J Surg Pathol. 1986 Sep;10(9):611–617. doi: 10.1097/00000478-198609000-00003. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Morén A., Severinsson L., Ek B., Ostman A., Betsholtz C., Heldin C. H. cDNA cloning and expression of a human platelet-derived growth factor (PDGF) receptor specific for B-chain-containing PDGF molecules. Mol Cell Biol. 1988 Aug;8(8):3476–3486. doi: 10.1128/mcb.8.8.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L. Platelet-derived growth factor receptor signals. J Biol Chem. 1994 Dec 23;269(51):32023–32026. [PubMed] [Google Scholar]
- Clark J. G., Madtes D. K., Raghu G. Effects of platelet-derived growth factor isoforms on human lung fibroblast proliferation and procollagen gene expression. Exp Lung Res. 1993 May-Jun;19(3):327–344. doi: 10.3109/01902149309064350. [DOI] [PubMed] [Google Scholar]
- Cohen B., Yoakim M., Piwnica-Worms H., Roberts T. M., Schaffhausen B. S. Tyrosine phosphorylation is a signal for the trafficking of pp85, an 85-kDa phosphorylated polypeptide associated with phosphatidylinositol kinase activity. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4458–4462. doi: 10.1073/pnas.87.12.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore P. A., Smith S. R. Growth factor effects on cells of the vascular wall: a survey. Growth Factors. 1993;8(1):61–75. doi: 10.3109/08977199309029135. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Forsberg K., Valyi-Nagy I., Heldin C. H., Herlyn M., Westermark B. Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):393–397. doi: 10.1073/pnas.90.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furcht L. T. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986 Nov;55(5):505–509. [PubMed] [Google Scholar]
- Gehlsen K. R., Dickerson K., Argraves W. S., Engvall E., Ruoslahti E. Subunit structure of a laminin-binding integrin and localization of its binding site on laminin. J Biol Chem. 1989 Nov 15;264(32):19034–19038. [PubMed] [Google Scholar]
- Gigi-Leitner O., Geiger B., Levy R., Czernobilsky B. Cytokeratin expression in squamous metaplasia of the human uterine cervix. Differentiation. 1986;31(3):191–205. doi: 10.1111/j.1432-0436.1986.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Guimond S., Maccarana M., Olwin B. B., Lindahl U., Rapraeger A. C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem. 1993 Nov 15;268(32):23906–23914. [PubMed] [Google Scholar]
- Hart C. E., Seifert R. A., Ross R., Bowen-Pope D. F. Synthesis, phosphorylation, and degradation of multiple forms of the platelet-derived growth factor receptor studied using a monoclonal antibody. J Biol Chem. 1987 Aug 5;262(22):10780–10785. [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Ostman A., Westermark B. Structure of platelet-derived growth factor: implications for functional properties. Growth Factors. 1993;8(4):245–252. doi: 10.3109/08977199308991570. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990 Jul;1(8):555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson M., Nistér M., Betsholtz C., Heldin C. H., Westermark B., Funa K. Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor (PDGF) B chain and PDGF receptor suggests autocrine growth stimulation. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7748–7752. doi: 10.1073/pnas.85.20.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S., Huang F., Friedman E. Platelet-derived growth factor-B increases colon cancer cell growth in vivo by a paracrine effect. J Cell Physiol. 1995 Nov;165(2):239–245. doi: 10.1002/jcp.1041650204. [DOI] [PubMed] [Google Scholar]
- Huang S. S., Huang J. S. Rapid turnover of the platelet-derived growth factor receptor in sis-transformed cells and reversal by suramin. Implications for the mechanism of autocrine transformation. J Biol Chem. 1988 Sep 5;263(25):12608–12618. [PubMed] [Google Scholar]
- Ito M., Yoshida K., Kyo E., Ayhan A., Nakayama H., Yasui W., Ito H., Tahara E. Expression of several growth factors and their receptor genes in human colon carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(3):173–178. doi: 10.1007/BF02899402. [DOI] [PubMed] [Google Scholar]
- Ivarsson M., Sundberg C., Farrokhnia N., Pertoft H., Rubin K., Gerdin B. Recruitment of type I collagen producing cells from the microvasculature in vitro. Exp Cell Res. 1996 Dec 15;229(2):336–349. doi: 10.1006/excr.1996.0379. [DOI] [PubMed] [Google Scholar]
- Kelly J. L., Sánchez A., Brown G. S., Chesterman C. N., Sleigh M. J. Accumulation of PDGF B and cell-binding forms of PDGF A in the extracellular matrix. J Cell Biol. 1993 Jun;121(5):1153–1163. doi: 10.1083/jcb.121.5.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Vlodavsky I. Biosynthesis and storage of basic fibroblast growth factor (bFGF) by endothelial cells: implication for the mechanism of action of angiogenesis. Prog Clin Biol Res. 1988;266:55–61. [PubMed] [Google Scholar]
- La Rocca R. V., Stein C. A., Myers C. E. Suramin: prototype of a new generation of antitumor compounds. Cancer Cells. 1990 Apr;2(4):106–115. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindmark G., Sundberg C., Glimelius B., Påhlman L., Rubin K., Gerdin B. Stromal expression of platelet-derived growth factor beta-receptor and platelet-derived growth factor B-chain in colorectal cancer. Lab Invest. 1993 Dec;69(6):682–689. [PubMed] [Google Scholar]
- Maccarana M., Casu B., Lindahl U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem. 1993 Nov 15;268(32):23898–23905. [PubMed] [Google Scholar]
- Miekka S. I., Ingham K. C., Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982 Jul 1;27(1):1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Miller F. R., McInerney D. Epithelial component of host-tumor interactions in the orthotopic site preference of a mouse mammary tumor. Cancer Res. 1988 Jul 1;48(13):3698–3701. [PubMed] [Google Scholar]
- Ostman A., Andersson M., Betsholtz C., Westermark B., Heldin C. H. Identification of a cell retention signal in the B-chain of platelet-derived growth factor and in the long splice version of the A-chain. Cell Regul. 1991 Jul;2(7):503–512. doi: 10.1091/mbc.2.7.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paku S., Paweletz N. First steps of tumor-related angiogenesis. Lab Invest. 1991 Sep;65(3):334–346. [PubMed] [Google Scholar]
- Pekny M., Ostman A., Hermansson A., Nistér M., Heldin C. H., Westermark B. Differences in binding to the solid substratum and extracellular matrix may explain isoform-specific paracrine effects of platelet-derived growth factor. Growth Factors. 1994;10(2):77–87. doi: 10.3109/08977199409010981. [DOI] [PubMed] [Google Scholar]
- Price J. E., Polyzos A., Zhang R. D., Daniels L. M. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990 Feb 1;50(3):717–721. [PubMed] [Google Scholar]
- Raines E. W., Ross R. Compartmentalization of PDGF on extracellular binding sites dependent on exon-6-encoded sequences. J Cell Biol. 1992 Jan;116(2):533–543. doi: 10.1083/jcb.116.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuterdahl C., Sundberg C., Rubin K., Funa K., Gerdin B. Tissue localization of beta receptors for platelet-derived growth factor and platelet-derived growth factor B chain during wound repair in humans. J Clin Invest. 1993 May;91(5):2065–2075. doi: 10.1172/JCI116429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuterdahl C., Tingström A., Terracio L., Funa K., Heldin C. H., Rubin K. Characterization of platelet-derived growth factor beta-receptor expressing cells in the vasculature of human rheumatoid synovium. Lab Invest. 1991 Mar;64(3):321–329. [PubMed] [Google Scholar]
- Risau W., Drexler H., Mironov V., Smits A., Siegbahn A., Funa K., Heldin C. H. Platelet-derived growth factor is angiogenic in vivo. Growth Factors. 1992;7(4):261–266. doi: 10.3109/08977199209046408. [DOI] [PubMed] [Google Scholar]
- Rönnstrand L., Terracio L., Claesson-Welsh L., Heldin C. H., Rubin K. Characterization of two monoclonal antibodies reactive with the external domain of the platelet-derived growth factor receptor. J Biol Chem. 1988 Jul 25;263(21):10429–10435. [PubMed] [Google Scholar]
- Rønnov-Jessen L., Petersen O. W., Koteliansky V. E., Bissell M. J. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995 Feb;95(2):859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Sitaras N. M., Antoniades H. N., Kufe D. W., Pantazis P. Expression of platelet-derived growth factor (PDGF)-related transcripts and synthesis of biologically active PDGF-like proteins by human malignant epithelial cell lines. J Clin Invest. 1988 Oct;82(4):1157–1164. doi: 10.1172/JCI113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Beitz J. G., Kato J., Yamamoto M., Clark J. W., Calabresi P., Raymond A., Frackelton A. R., Jr Platelet-derived growth factor indirectly stimulates angiogenesis in vitro. Am J Pathol. 1993 Apr;142(4):1119–1130. [PMC free article] [PubMed] [Google Scholar]
- Schlingemann R. O., Dingjan G. M., Emeis J. J., Blok J., Warnaar S. O., Ruiter D. J. Monoclonal antibody PAL-E specific for endothelium. Lab Invest. 1985 Jan;52(1):71–76. [PubMed] [Google Scholar]
- Seemayer T. A., Schürch W., Lagacé R. Myofibroblasts in human pathology. Hum Pathol. 1981 Jun;12(6):491–492. doi: 10.1016/s0046-8177(81)80061-5. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Shiraishi T., Morimoto S., Itoh K., Sato H., Sugihara K., Onishi T., Ogihara T. Radioimmunoassay of human platelet-derived growth factor using monoclonal antibody toward a synthetic 73-97 fragment of its B-chain. Clin Chim Acta. 1989 Sep 15;184(1):65–74. doi: 10.1016/0009-8981(89)90257-x. [DOI] [PubMed] [Google Scholar]
- Siegbahn A., Hammacher A., Westermark B., Heldin C. H. Differential effects of the various isoforms of platelet-derived growth factor on chemotaxis of fibroblasts, monocytes, and granulocytes. J Clin Invest. 1990 Mar;85(3):916–920. doi: 10.1172/JCI114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors and inflammation, tissue repair, and cancer. J Clin Invest. 1986 Aug;78(2):329–332. doi: 10.1172/JCI112580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg C., Ljungström M., Lindmark G., Gerdin B., Rubin K. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am J Pathol. 1993 Nov;143(5):1377–1388. [PMC free article] [PubMed] [Google Scholar]
- Sundberg C., Rubin K. Stimulation of beta1 integrins on fibroblasts induces PDGF independent tyrosine phosphorylation of PDGF beta-receptors. J Cell Biol. 1996 Feb;132(4):741–752. doi: 10.1083/jcb.132.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracio L., Rönnstrand L., Tingström A., Rubin K., Claesson-Welsh L., Funa K., Heldin C. H. Induction of platelet-derived growth factor receptor expression in smooth muscle cells and fibroblasts upon tissue culturing. J Cell Biol. 1988 Nov;107(5):1947–1957. doi: 10.1083/jcb.107.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingström A., Reuterdahl C., Lindahl P., Heldin C. H., Rubin K. Expression of platelet-derived growth factor-beta receptors on human fibroblasts. Regulation by recombinant platelet-derived growth factor-BB, IL-1, and tumor necrosis factor-alpha. J Immunol. 1992 Jan 15;148(2):546–554. [PubMed] [Google Scholar]
- Vassbotn F. S., Ostman A., Langeland N., Holmsen H., Westermark B., Heldin C. H., Nistér M. Activated platelet-derived growth factor autocrine pathway drives the transformed phenotype of a human glioblastoma cell line. J Cell Physiol. 1994 Feb;158(2):381–389. doi: 10.1002/jcp.1041580221. [DOI] [PubMed] [Google Scholar]
- Webb D. S., Shimizu Y., Van Seventer G. A., Shaw S., Gerrard T. L. LFA-3, CD44, and CD45: physiologic triggers of human monocyte TNF and IL-1 release. Science. 1990 Sep 14;249(4974):1295–1297. doi: 10.1126/science.1697984. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M., Moll R., Wiklund B., Lüning B. Tissue polypeptide antigen (TPA) is related to the non-epidermal keratins 8, 18 and 19 typical of simple and non-squamous epithelia: re-evaluation of a human tumor marker. EMBO J. 1984 Nov;3(11):2707–2714. doi: 10.1002/j.1460-2075.1984.tb02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark B., Heldin C. H. Platelet-derived growth factor in autocrine transformation. Cancer Res. 1991 Oct 1;51(19):5087–5092. [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]








