Abstract
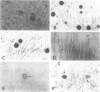
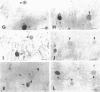
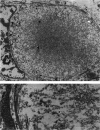
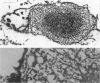
To clarify the pathological characteristics of astrocytic hyaline inclusions (Ast-HIs) in patients with familial amyotrophic lateral sclerosis (FALS) with neuronal Lewy-body-like hyaline inclusions (LBHIs), eight autopsies on members of four different families, including two long-term surviving patients with clinical courses of over 10 years, were analyzed. Ast-HIs were found only in the two long-term surviving patients who belonged to different families and to different races. Ast-HIs were ultrastructurally composed of 15- to 25-nm granule-coated fibrils that had immunoreactivities to superoxide dismutase 1 (SOD1) and ubiquitin. Approximately 50% of the Ast-HIs expressed alpha B-crystallin, metallothionein, glutamine synthetase, and tubulin (alpha and beta) at various intensities. Some Ast-HIs reacted with antibodies to tau protein, S-100 protein, and heat shock protein 27. The Ast-HIs were not stained for glial fibrillary acidic protein. Our results suggest a cooperative role of superoxide dismutase 1, ubiquitin, and cytoskeletal proteins in the formation of granule-coated fibrils (namely, Ast-HIs) and provide evidence that Ast-HIs are formed in certain long-surviving familial amyotrophic lateral sclerosis patients with neuronal Lewy-body-like hyaline inclusions.
Full text
PDF


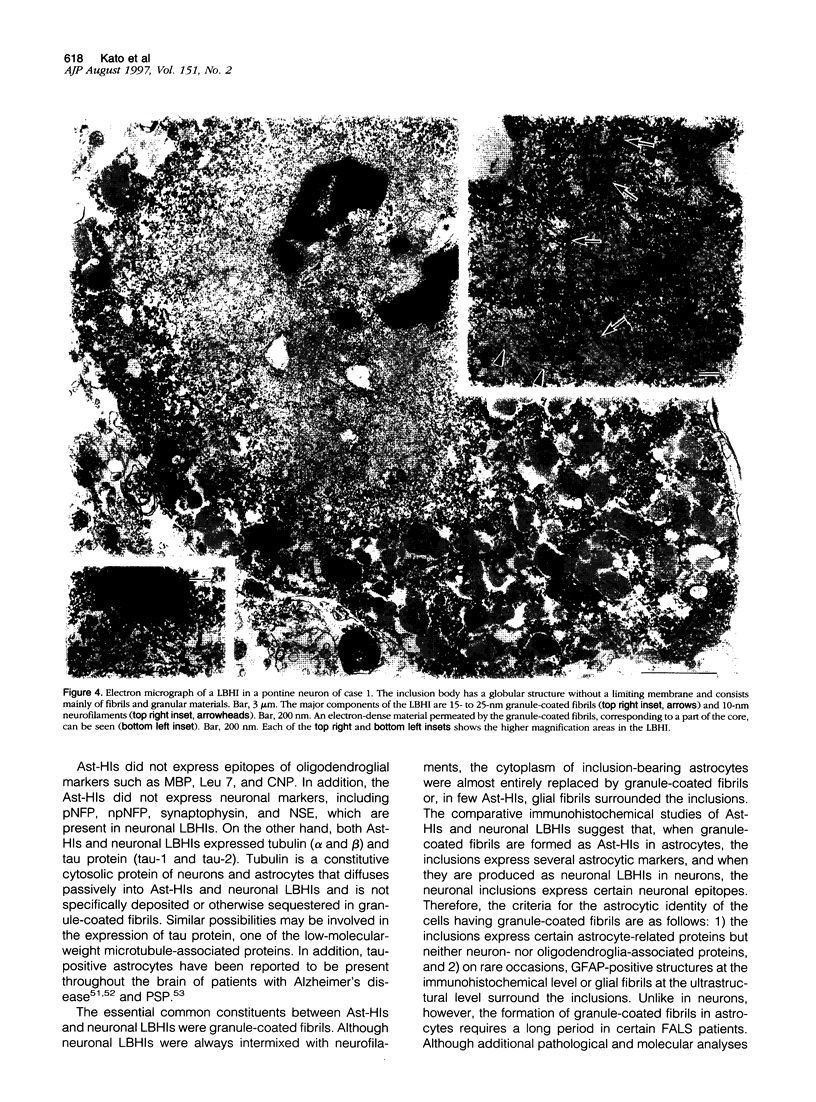


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. M., Nilsson P., Ala-Hurula V., Keränen M. L., Tarvainen I., Haltia T., Nilsson L., Binzer M., Forsgren L., Marklund S. L. Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nat Genet. 1995 May;10(1):61–66. doi: 10.1038/ng0595-61. [DOI] [PubMed] [Google Scholar]
- Asayama K., Burr I. M. Joint purification of mangano and cuprozinc superoxide dismutases from a single source--a simplified method. Anal Biochem. 1984 Feb;136(2):336–339. doi: 10.1016/0003-2697(84)90226-4. [DOI] [PubMed] [Google Scholar]
- Asayama K., Janco R. L., Burr I. M. Selective induction of manganous superoxide dismutase in human monocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):C393–C397. doi: 10.1152/ajpcell.1985.249.5.C393. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauwgeers H. G., Sillevis Smitt P. A., De Jong J. M., Troost D. Distribution of metallothionein in the human central nervous system. Glia. 1993 May;8(1):62–70. doi: 10.1002/glia.440080108. [DOI] [PubMed] [Google Scholar]
- Bühler R. H., Kägi J. H. Human hepatic metallothioneins. FEBS Lett. 1974 Feb 15;39(2):229–234. doi: 10.1016/0014-5793(74)80057-8. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Laing N., Hurse P. V., Brown R. H., Jr Toxic mutants in Charcot's sclerosis. Nature. 1995 Nov 23;378(6555):342–343. doi: 10.1038/378342a0. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Gurney M. E. Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am J Pathol. 1994 Dec;145(6):1271–1279. [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Gurney M. E. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res. 1995 Apr 3;676(1):25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Tainer J. A., Mitsumoto H., Ohnishi A., He X., Hung W. Y., Zhao Y., Juneja T., Hentati A., Siddique T. Two novel SOD1 mutations in patients with familial amyotrophic lateral sclerosis. Hum Mol Genet. 1995 Jun;4(6):1113–1116. doi: 10.1093/hmg/4.6.1113. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Ogura T., Hokari A., Weisz A., Yamashita J., Esumi H. Inducible nitric oxide synthase in a human glioblastoma cell line. J Neurochem. 1995 Jan;64(1):85–91. doi: 10.1046/j.1471-4159.1995.64010085.x. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hershko A. Ubiquitin: roles in protein modification and breakdown. Cell. 1983 Aug;34(1):11–12. doi: 10.1016/0092-8674(83)90131-9. [DOI] [PubMed] [Google Scholar]
- Hirano A., Kurland L. T., Sayre G. P. Familial amyotrophic lateral sclerosis. A subgroup characterized by posterior and spinocerebellar tract involvement and hyaline inclusions in the anterior horn cells. Arch Neurol. 1967 Mar;16(3):232–243. doi: 10.1001/archneur.1967.00470210008002. [DOI] [PubMed] [Google Scholar]
- Hirano A., Nakano I., Kurland L. T., Mulder D. W., Holley P. W., Saccomanno G. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984 Sep;43(5):471–480. doi: 10.1097/00005072-198409000-00002. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Iwaki A., Miyazono M., Goldman J. E. Preferential expression of alpha B-crystallin in astrocytic elements of neuroectodermal tumors. Cancer. 1991 Nov 15;68(10):2230–2240. doi: 10.1002/1097-0142(19911115)68:10<2230::aid-cncr2820681023>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- KURLAND L. T., MULDER D. W. Epidemiologic investigations of amyotrophic lateral sclerosis. 2. Familial aggregations indicative of dominant inheritance. II. Neurology. 1955 Apr;5(4):249–268. doi: 10.1212/wnl.5.4.249. [DOI] [PubMed] [Google Scholar]
- Kato M., Herz F., Kato S., Hirano A. Expression of stress-response (heat-shock) protein 27 in human brain tumors: an immunohistochemical study. Acta Neuropathol. 1992;83(4):420–422. doi: 10.1007/BF00713535. [DOI] [PubMed] [Google Scholar]
- Kato S., Hirano A., Kato M., Herz F., Ohama E. Comparative study on the expression of stress-response protein (srp) 72, srp 27, alpha B-crystallin and ubiquitin in brain tumours. An immunohistochemical investigation. Neuropathol Appl Neurobiol. 1993 Oct;19(5):436–442. doi: 10.1111/j.1365-2990.1993.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Kato S., Morita T., Hori T., Kato M., Hirano A., Herz F., Ohama E. Brain tumor: immunohistochemical studies on the stress-response proteins, p53 protein and proliferating cell nuclear antigen. Noshuyo Byori. 1995;12(2):125–132. [PubMed] [Google Scholar]
- Kato S., Shimoda M., Watanabe Y., Nakashima K., Takahashi K., Ohama E. Familial amyotrophic lateral sclerosis with a two base pair deletion in superoxide dismutase 1: gene multisystem degeneration with intracytoplasmic hyaline inclusions in astrocytes. J Neuropathol Exp Neurol. 1996 Oct;55(10):1089–1101. [PubMed] [Google Scholar]
- Kato T., Hirano A., Kurland L. T. Asymmetric involvement of the spinal cord involving both large and small anterior horn cells in a case of familial amyotrophic lateral sclerosis. Clin Neuropathol. 1987 Mar-Apr;6(2):67–70. [PubMed] [Google Scholar]
- Kenessey A., Yen S. H. The extent of phosphorylation of fetal tau is comparable to that of PHF-tau from Alzheimer paired helical filaments. Brain Res. 1993 Nov 26;629(1):40–46. doi: 10.1016/0006-8993(93)90478-6. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Nishizawa Y., Takahashi Y., Odani S. Chemical, immunological and catalytic properties of 2':3'-cyclic nucleotide 3'-phosphodiesterase purified from brain white matter. Biochem J. 1981 Apr 1;195(1):153–157. doi: 10.1042/bj1950153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi J. H. Overview of metallothionein. Methods Enzymol. 1991;205:613–626. doi: 10.1016/0076-6879(91)05145-l. [DOI] [PubMed] [Google Scholar]
- Lee S., Park Y. D., Yen S. H., Ksiezak-Reding H., Goldman J. E., Dickson D. W. A study of infantile motor neuron disease with neurofilament and ubiquitin immunocytochemistry. Neuropediatrics. 1989 May;20(2):107–111. doi: 10.1055/s-2008-1071275. [DOI] [PubMed] [Google Scholar]
- Matus A., Mughal S. Immunohistochemical localisation of S-100 protein in brain. Nature. 1975 Dec 25;258(5537):746–748. doi: 10.1038/258746a0. [DOI] [PubMed] [Google Scholar]
- Metcalf C. W., Hirano A. Amyotrophic lateral sclerosis. Clinicopathological studies of a family. Arch Neurol. 1971 Jun;24(6):518–523. doi: 10.1001/archneur.1971.00480360052006. [DOI] [PubMed] [Google Scholar]
- Mizusawa H., Matsumoto S., Yen S. H., Hirano A., Rojas-Corona R. R., Donnenfeld H. Focal accumulation of phosphorylated neurofilaments within anterior horn cell in familial amyotrophic lateral sclerosis. Acta Neuropathol. 1989;79(1):37–43. doi: 10.1007/BF00308955. [DOI] [PubMed] [Google Scholar]
- Murayama S., Ookawa Y., Mori H., Nakano I., Ihara Y., Kuzuhara S., Tomonaga M. Immunocytochemical and ultrastructural study of Lewy body-like hyaline inclusions in familial amyotrophic lateral sclerosis. Acta Neuropathol. 1989;78(2):143–152. doi: 10.1007/BF00688202. [DOI] [PubMed] [Google Scholar]
- Nakano I., Iwatsubo T., Otsuka N., Kamei M., Matsumura K., Mannen T. Paired helical filaments in astrocytes: electron microscopy and immunohistochemistry in a case of atypical Alzheimer's disease. Acta Neuropathol. 1992;83(3):228–232. doi: 10.1007/BF00296783. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Watanabe Y., Kuno N., Nanba E., Takahashi K. Abnormality of Cu/Zn superoxide dismutase (SOD1) activity in Japanese familial amyotrophic lateral sclerosis with two base pair deletion in the SOD1 gene. Neurology. 1995 May;45(5):1019–1020. doi: 10.1212/wnl.45.5.1019-a. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Nishimura N., Tohyama C. Immunohistochemical localization of metallothionein in developing rat tissues. J Histochem Cytochem. 1989 May;37(5):715–722. doi: 10.1177/37.5.2703706. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Namba Y., Ikeda K., Oda M. Glial fibrillary tangles with straight tubules in the brains of patients with progressive supranuclear palsy. Neurosci Lett. 1992 Aug 31;143(1-2):35–38. doi: 10.1016/0304-3940(92)90227-x. [DOI] [PubMed] [Google Scholar]
- Ohshima H., Oguchi S., Adachi H., Iida S., Suzuki H., Sugimura T., Esumi H. Purification of nitric oxide synthase from bovine brain: immunological characterization and tissue distribution. Biochem Biophys Res Commun. 1992 Feb 28;183(1):238–244. doi: 10.1016/0006-291x(92)91634-3. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C. Tau protein immunoreactivity in dementia of the Alzheimer type. I. Morphology, evolution, distribution, and pathogenetic implications. Lab Invest. 1989 Jan;60(1):123–137. [PubMed] [Google Scholar]
- Papasozomenos S. C. Tau protein immunoreactivity in dementia of the Alzheimer type: II. Electron microscopy and pathogenetic implications. Effects of fixation on the morphology of the Alzheimer's abnormal filaments. Lab Invest. 1989 Mar;60(3):375–389. [PubMed] [Google Scholar]
- Pramatarova A., Goto J., Nanba E., Nakashima K., Takahashi K., Takagi A., Kanazawa I., Figlewicz D. A., Rouleau G. A. A two basepair deletion in the SOD 1 gene causes familial amyotrophic lateral sclerosis. Hum Mol Genet. 1994 Nov;3(11):2061–2062. [PubMed] [Google Scholar]
- Renkawek K., de Jong W. W., Merck K. B., Frenken C. W., van Workum F. P., Bosman G. J. alpha B-crystallin is present in reactive glia in Creutzfeldt-Jakob disease. Acta Neuropathol. 1992;83(3):324–327. doi: 10.1007/BF00296796. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothschild B. M., Hershkovitz I., Rothschild C. Origin of yaws in the Pleistocene. Nature. 1995 Nov 23;378(6555):343–344. doi: 10.1038/378343b0. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Amyotrophic lateral sclerosis: human challenge for neuroscience. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1251–1253. doi: 10.1073/pnas.92.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Hirano A., Kobayashi M., Siddique T., Deng H. X., Hung W. Y., Kato T., Asayama K. Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J Neuropathol Exp Neurol. 1996 Apr;55(4):481–490. doi: 10.1097/00005072-199604000-00011. [DOI] [PubMed] [Google Scholar]
- Siddique T., Pericak-Vance M. A., Brooks B. R., Roos R. P., Hung W. Y., Antel J. P., Munsat T. L., Phillips K., Warner K., Speer M. Linkage analysis in familial amyotrophic lateral sclerosis. Neurology. 1989 Jul;39(7):919–925. doi: 10.1212/wnl.39.7.919. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Makifuchi T., Nakano R., Sato S., Inuzuka T., Sakimura K., Mishina M., Honma Y., Tsuji S., Ikuta F. Familial amyotrophic lateral sclerosis with a mutation in the Cu/Zn superoxide dismutase gene. Acta Neuropathol. 1994;88(2):185–188. doi: 10.1007/BF00294513. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nakamura H., Okada E. Hereditary amyotrophic lateral sclerosis. Histochemical and electron microscopic study of hyaline inclusions in motor neurons. Arch Neurol. 1972 Oct;27(4):292–299. doi: 10.1001/archneur.1972.00490160020003. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Nakamura H., Tabuchi Y., Takahashi K. Familial amyotrophic lateral sclerosis: features of multisystem degeneration. Acta Neuropathol. 1984;64(1):22–29. doi: 10.1007/BF00695602. [DOI] [PubMed] [Google Scholar]
- Wiedau-Pazos M., Goto J. J., Rabizadeh S., Gralla E. B., Roe J. A., Lee M. K., Valentine J. S., Bredesen D. E. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996 Jan 26;271(5248):515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- Yamada T., Calne D. B., Akiyama H., McGeer E. G., McGeer P. L. Further observations on Tau-positive glia in the brains with progressive supranuclear palsy. Acta Neuropathol. 1993;85(3):308–315. doi: 10.1007/BF00227727. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Crowe A., Dickson D. W. Monoclonal antibodies to Alzheimer neurofibrillary tangles. 1. Identification of polypeptides. Am J Pathol. 1985 Aug;120(2):282–291. [PMC free article] [PubMed] [Google Scholar]
- de Belleroche J., Orrell R. W., Virgo L. Amyotrophic lateral sclerosis: recent advances in understanding disease mechanisms. J Neuropathol Exp Neurol. 1996 Jul;55(7):747–757. [PubMed] [Google Scholar]