Abstract
Monkeys are able to discriminate the difference in frequency between two periodic mechanical vibrations applied sequentially to the fingertips. It has been proposed that this ability is mediated by the periodicity of the responses in the quickly adapting (QA) neurons of the primary somatosensory cortex (S1), instead of the average firing rates. We recorded from QA neurons of S1 while monkeys performed the vibrotactile discrimination task. We found that the periodic mechanical vibrations can be represented both in the periodicity and in the firing rate responses to varying degrees across the QA neuronal population. We then computed neurometric functions by using both the periodicity and the firing rate and sought to determine which of these two measures is associated with the psychophysical performance. We found that neurometric thresholds based on the firing rate are very similar to the animal's psychometric thresholds whereas neurometric thresholds based on periodicity are far lower than those thresholds. These results indicate that an observer could solve this task with a precision similar to that of the monkey, based only on the firing rate produced during the stimulus periods.
A fundamental issue in neurobiology is understanding precisely which component of the neuronal activity evoked by a sensory stimulus is meaningful for perception (1–3). This has been investigated in monkeys trained to discriminate among vibrotactile stimuli (4–11), and it has been proposed that an observer could solve this task by measuring the periodic, neuronal spike intervals resulting from vibrotactile stimuli, instead of the firing rate in the quickly adapting (QA) neurons of the primary somatosensory cortex (S1) (5, 7, 8). However, this proposal is based on the study of a small number of QA neurons of S1 (7) or on responses to a narrow range of frequencies applied to anesthetized animals (8). Recently, we observed that monkeys can discriminate the mean frequencies of aperiodic stimuli, which lack any temporal regularity (10). Psychometric thresholds for periodic and aperiodic stimulus discrimination are very similar. Additionally, animals discriminate periodic and aperiodic stimuli whether these are delivered naturally, by a mechanical probe to the fingertips, or artificially, through microinjection of electrical current frequencies into the QA circuit of S1 (10, 11). These results challenge the proposal that periodicity is the neural signal used by monkeys to perform frequency discrimination (5, 7). To investigate this issue further, we recorded from a large number of single QA neurons in S1 while monkeys performed the vibrotactile discrimination task (6, 7–11), measuring both the periodic spike intervals and the firing rate during the stimulus periods. We found that the periodic mechanical vibrations can be represented both in the periodicity and in the firing rate responses to varying degrees across the QA neuronal population. We then computed neurometric functions by using both periodicity and firing rate and sought to determine which of these two measures is associated with the psychophysical performance. We found that neurometric thresholds based on the firing rate are very similar to the animal's psychometric thresholds whereas neurometric thresholds based on periodicity are far lower than those thresholds. These results indicate that an observer could solve this behavioral task with a precision similar to that of the monkey, based only on the average firing rate produced during the stimulus periods, and without regard for the periodic responses of some neurons.
Methods
General.
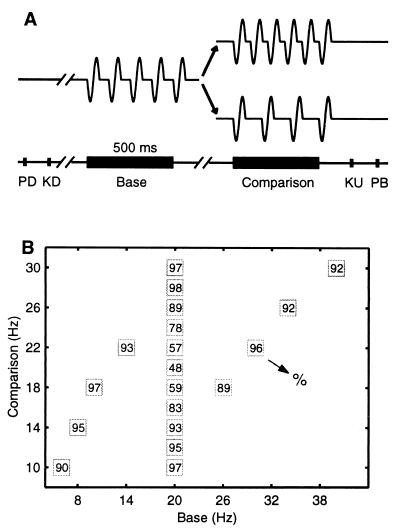
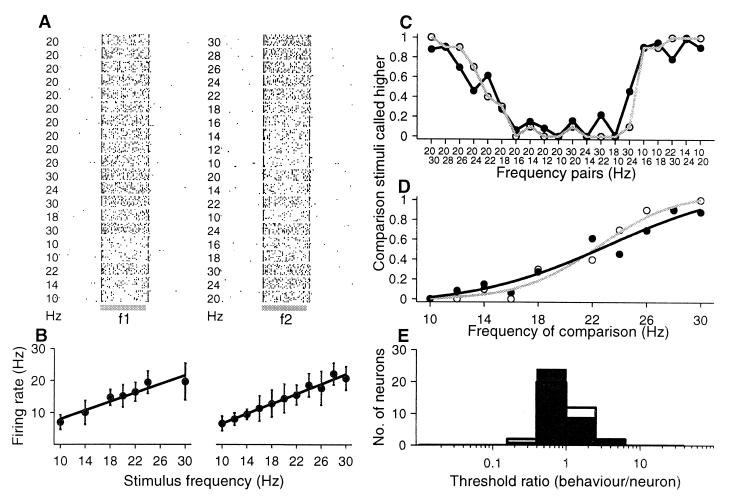
Four monkeys (Macaca mulatta) were trained to discriminate the difference in frequency between two mechanical vibrations delivered sequentially to their fingertips (9–11), and they learned to indicate whether the second frequency was higher or lower than the first (Fig. 1A). Neurophysiological recordings were made in S1 (areas 3b and 1) contralateral to the mechanical stimulation while monkeys performed the discrimination task (7, 10–12). The neurons selected for study in S1 had small, cutaneous receptive fields confined to the smooth, hairless skin of one fingertip of digits 2, 3, or 4. All neurons had QA properties. The neuronal responses from S1 were collected while monkeys discriminated frequencies at psychophysical thresholds (Fig. 1B).
Figure 1.
Discrimination task. (A) Sequence of events during discrimination trials. The mechanical probe is lowered, indenting the glabrous skin of one digit of the hand (PD); the monkey places his free hand on an immovable key (KD); the probe oscillates vertically, at the base frequency; after a delay, a second mechanical vibration is delivered at the comparison frequency; the monkey releases the key (KU) and presses one of two push-buttons (PB) to indicate whether the comparison frequency was higher or lower than the base. (B) Stimulus set used during recording. Each box indicates a base frequency/comparison frequency stimulus pair used; the number inside the box indicates overall percent correct trials for that base/comparison pair.
Discrimination Task.
The discrimination task used here was described before (7, 9–11), but, in brief, stimuli were delivered to the skin of the distal segments of one digit of the right, restrained hand, via a computer-controlled Chubbuck motor stimulator (BME Systems, Baltimore; 2-mm round tip). The initial indentation was 500 μm. Vibrotactile stimuli were trains of short mechanical pulses. Each of these pulses consisted of single cycle sinusoid lasting 20 ms. Stimulus amplitudes were adjusted to equal subjective intensities (7, 9): for example, 71 μm at 12 Hz and 51 μm at 34 Hz (≈1.4% per Hz). During trials, two vibrotactile stimuli were delivered consecutively to the glabrous (hairless) skin, separated by an interstimulus delay of 1–3 s, and the animal was rewarded for correct discrimination with a drop of liquid. Discrimination was indicated by pressing one of two push-buttons. Performance was measured through psychometric techniques (7, 9–11). Animals were handled in accordance with the institutional standards of the National Institutes of Health and the Society for Neuroscience.
Recording Sessions and Sites.
Neuronal recordings were obtained with an array of seven independent, moveable microelectrodes (7, 10–12) (2–3 MΩ inserted into S1 (areas 3b and 1; four monkeys). Recording sites changed from session to session, and standard histological procedures were used to construct surface maps of all of the penetrations in S1. This was done first by marking the edges of the small chambers (7 mm in diameter) placed above S1. Additionally, in the last recording sessions, we made small lesions at different depths in the recording area. We considered neurons recorded in area 1, from the top of the cortex to 2,500 μm, and in area 3b, from 2,500 μm down. All of these neurons had small cutaneous receptive fields confined to the distal segments of fingertips 2, 3, or 4, and had QA properties.
Data Analysis.
For each neuron studied during the discrimination task, off-line analysis and statistical tests were done by using custom and matlab software (Mathworks, Natick, MA). The analysis was restricted to the stimulus periods, according to two criteria. First, we devised a measure that quantified the capacity of the neurons to represent the periodicity of the stimulus. For each trial, the power spectrum of the spike train evoked during the stimulus period was computed (fast Fourier transform, n = 216; sampling frequency, 10 kHz; resolution, 0.15 Hz; range, 6–100 Hz) (13). As an estimate of the periodicity, we calculated the median frequency around the peak power spectrum frequency, weighted according to the power at each frequency. The frequencies used for this measure were limited to those within a factor of 1.8 of the peak frequency (to avoid contamination by harmonics) and to frequencies with a power greater than 15% of the peak power (to avoid noise). The median frequency calculated in this way was considered a quantitative measure of periodicity evoked in S1 neurons by the periodic mechanical stimuli. Second, for each trial, we calculated the mean firing rate over the stimulus periods. For each stimulus frequency, we computed the mean ± SD of both periodicity and firing rate over all trials with that stimulus frequency. For further analysis, we selected those neurons that had the best linear fit (χ2, Q > 0.05) of the periodicity and/or firing rate values as a function of the stimulus frequency (13). We also required the slope of this linear fit to be significantly different from zero (permutation test, n = 1,000, P < 0.05) (14), and that the slopes calculated separately for each the two stimulus periods (base and comparison) were not significantly different from each other (α < 0.05 as computed by using the SD of the linear fits) (15).
The discrimination task requires the comparison of the second stimulus frequency against the first. We observed that QA neurons of S1 provide a reliable representation of the two stimulus frequencies. We then determined the probability that an observer (a cortical region central to S1) could distinguish the difference between the two stimuli. This could be based on a comparison of the neuronal response distributions of the second stimulus frequency (f2) made against the neuronal response distributions of the first stimulus frequency (f1). According to this, the observer could use a simple rule: if the number of spikes during the second stimulus is higher than during the first stimulus, then f2 is higher than f1. The same rule can be used when considering the periodicity values: if the periodicity values during the second stimulus period (f2) are higher than during the first stimulus (f1), then f2 is higher than f1 (16). This rule can be tested by determining the area under the curve receiver operating characteristic (ROC) generated by the neuronal response distributions for each pair of stimulus frequencies, using both periodicity and firing rate values (16). In pairs of stimulus frequencies in which the neuronal response distributions of f2 are much higher than the neuronal response distributions of f1, ROC values are close to 1; if the neuronal response distributions of f2 are much lower than the neuronal response distributions of f1, ROC values are close to 0; for overlapping distributions, intermediate ROC values are found. The ROC values were then used to compute neurometric functions. Psychophysical and neuronal discrimination thresholds were calculated as half of the difference between the stimulus frequency identified as higher than the base in 75% of the trials and that frequency identified as higher in 25% of the trials (6, 8). These were read directly from the logistic functions (Boltzmann's equation) expressed in terms of hertz.
Results
We first determined the responses of 223 QA neurons of S1 (135 in area 3b and 88 in area 1) as a function of the stimulus frequency while monkeys performed the discrimination task (Figs. 3A and 4A show examples of two types of responses). This was done by measuring for each neuron the periodicity and the mean firing rate during the stimulus periods in both single trials and in blocks of the same trials (see Methods). The results of these two measures indicate that the stimulus frequency can be represented both in the periodicity and in the firing rate responses to varying degrees across the QA neuronal population (Fig. 2). We were able to identify 188 neurons (113 in area 3b and 75 in area 1) that gave information about the stimulus frequency. One hundred and thirty-nine neurons responded with periodic spike intervals (Fig. 3 A and B) at a frequency that reliably represented the input stimulus frequency (94 in area 3b and 45 in area 1), and 72 other neurons increased their firing rate (Fig. 4 A and B) as a function of the stimulus frequency (49 in area 3b and 23 in area 1). Only 23 of the neurons (14 in area 3b and 9 in area 1) provided information about the stimulus frequency in terms of both periodicity and mean firing rate. This analysis, therefore, established the relationship between the neuronal responses and the stimulus frequency while monkeys performed the discrimination task. It is important to remark that previous studies had not reported neurons with aperiodic, stimulus-dependent firing rate responses (7, 8).
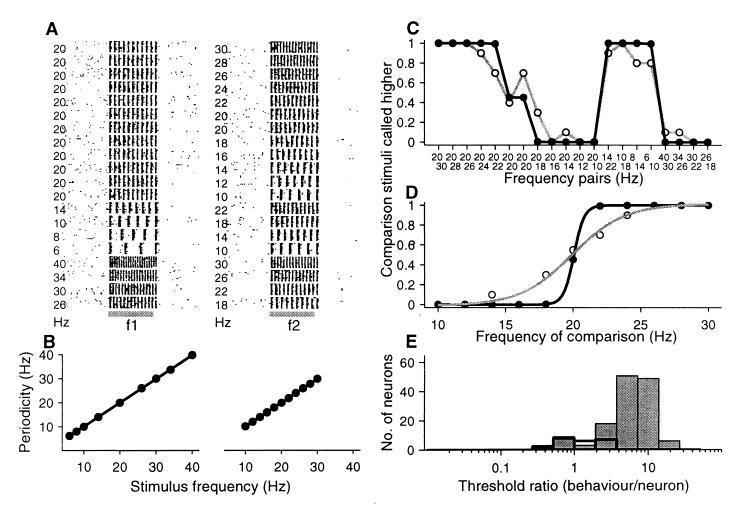
Figure 3.
Periodic responses of an area 1 neuron during the discrimination task. (A) Raster plots. Each row of ticks represents a trial, and each tick represents an action potential. Trials were randomly delivered. Gray horizontal lines indicate the first (f1) and the second (f2) stimulus. (B) Periodicity (± SD) as a function of the first and second stimulus frequencies. (C) Relationship between psychometric and neurometric functions. This is plotted as the probability that the second stimulus is judged higher than the first. (D) Psychometric and neurometric discrimination functions; data and sigmoidal fits (χ2 test, P < 0.001) for eleven pairs of stimulus frequencies in which the base frequency was 20 Hz. In C and D, gray lines represent psychometric functions; black ones neurometric functions. (E) Threshold ratios (psychometric/neurometric thresholds) calculated from neurons with periodic responses (gray bars). Open bars represent the threshold ratios between psychometric and neurometric thresholds calculated from a small number of neurons with modulations in their firing rate.
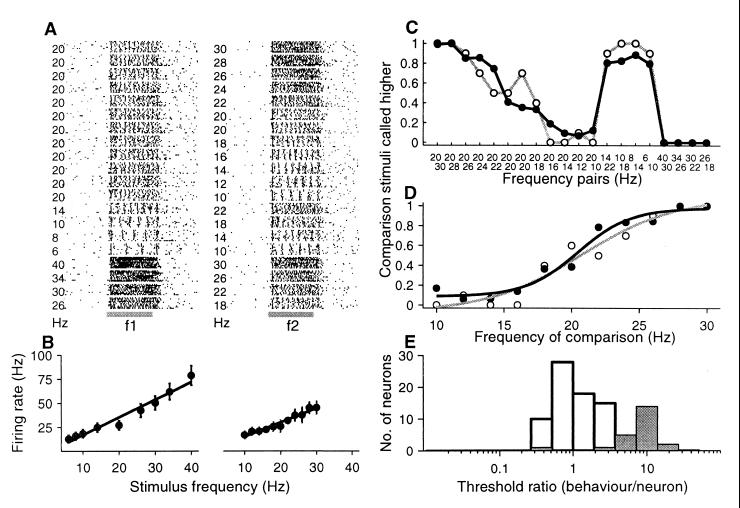
Figure 4.
Firing rate modulation of an area 3b neuron during the discrimination task. Same format as Fig. 3A. (A) Raster plots. (B) Mean rate (± SD) as a function of the stimulus frequency. (C) Relationship between psychometric and neurometric functions. (D) Psychometric and neurometric functions. (E) Threshold ratios calculated between psychometric and neurometric thresholds for each neuron, which varied the firing rate as a function of the stimulus frequency (open bars). Gray bars represent the threshold ratios between psychometric and neurometric thresholds calculated from a small number of neurons that show periodicity.
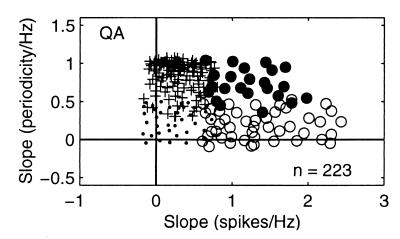
Figure 2.
Response properties of QA neurons of S1 as a function of the stimulus frequency. For each neuron studied during the frequency discrimination task, we calculated the slope of the best linear fit of the periodicity and/or firing rate values as a function of the stimulus frequencies. We required a good fit (χ2, Q > 0.05) and the slope of this linear fit to be significantly different from zero (permutation test, n = 1,000, P < 0.01). Each data point corresponds to the intersection of the slopes of periodicity (y axes) vs. firing rate (x axes). Plotting periodicity vs. firing rate for each neuron studied during the discrimination task gave different clusters of response patterns. Small dots are neurons that did not provide information about the stimulus frequency in terms of both periodicity and firing rate. Pluses are neurons that gave information about the stimulus frequency in periodicity only. Black circles are neurons that provided information about the stimulus frequency in terms of both periodicity and in the firing rate. Open circles are the neurons that provided information about the stimulus frequency in the firing rate only.
Having quantified the responses of S1 neurons as a function of the stimulus frequency, we proceeded to determine whether these neural signals carry physiological information that might be associated with psychophysical behavior. For each neuron, we computed neurometric functions by using the periodic or the firing rate values (see Methods). We first focused our attention on those neurons that responded with periodic spike intervals at a frequency of the input stimulus. Fig. 3A shows the responses of a S1 neuron during the two stimulus periods while the monkey discriminated between pairs of frequencies. The responses of this neuron matched the input stimulus frequency (Fig. 3B). The question is then whether in the periodic spike intervals a neural signal is to be found that matches the animal's psychophysical behavior. Fig. 3C shows the strong relationship between the psychometric and neurometric functions whereas Fig. 3D shows that the psychometric threshold (2.08 Hz) is higher than the neurometric threshold (0.20 Hz); the psychometric/neurometric threshold ratio = 10.4. Fig. 3E shows the psychometric/neurometric threshold ratios [6.53 ± 3.87 (mean ± SD); gray bars] over the population of periodic neurons, and it is clear that, based on response periodicity, these neurons discriminate vibrotactile stimuli (neurometric threshold = 0.79 Hz ± 1.22 Hz) much better than the animals do (psychometric threshold = 2.95 ± 1.87 Hz).
As indicated above, some QA neurons of S1 modulate their firing rate as a function of the increasing stimulus frequency (Fig. 4 A and B). Are these neural signals associated with the animal's psychophysical behavior? We computed neurometric functions for each of these neurons by using the firing rate values (see Methods). Fig. 4C shows the relationship between the psychometric and neurometric functions for an example neuron. The neurometric threshold (2.48 Hz) computed from this neuron (Fig. 4D) is slightly lower than the animal's psychometric threshold (3.22 Hz); the psychometric/neurometric threshold ratio = 1.29. Fig. 4E (open bars) shows the relationship between the psychometric (3.07 ± 0.34 Hz) and neurometric (3.37 ± 1.82 Hz) thresholds for the population of modulated firing rate neurons; the psychometric/neurometric threshold ratio = 1.31 ± 0.94.
It is clear from these two measures that neurometric thresholds based on periodicity are far lower than the psychometric thresholds whereas neurometric functions based on mean firing rate are very close to the psychometric thresholds.
A minority of neurons (23 of 188) provided information about the stimulus in both of their periodic spike intervals (the neurometric threshold = 1.31 ± 0.94 Hz; the psychometric/neurometric threshold ratio = 8.99 ± 3.68; gray bars of Fig. 4E), and in their mean firing rate (the neurometric threshold = 2.95 ± 1.87 Hz; the psychometric/neurometric threshold ratio = 1.56 ± 1.0; open bars of Fig. 4E). Once again, for this subpopulation of neurons, psychometric thresholds are far higher than neurometric thresholds based on periodicity, but are similar to neurometric ratios based on firing rate.
It has been proposed that the discrimination of vibrotactile stimuli depends on the periodic spike structure of the spike trains evoked in S1 (5–8). However, monkeys are able to discriminate aperiodic stimulus frequencies delivered to the fingertips or artificially injected in S1 (10). We studied 36 S1 neurons in each of two conditions: while monkeys discriminated between periodic stimuli, and while monkeys discriminated between aperiodic stimuli. Eleven of these neurons gave information about the periodic stimulus frequency in both periodicity and firing rate whereas the rest (25 neurons) gave information only in the firing rate. All 36 neurons gave information about the aperiodic stimulus frequency in their firing rate. (Because of the aperiodic stimulus design, even highly stimulus-entrained neurons do not carry information about stimulus frequency in their periodicity). Fig. 5A shows the responses of a recorded neuron in area 1 during the discrimination of aperiodic mean stimulus frequencies. Fig. 5B shows that the mean firing rate increases as a function of the stimulus frequency. Fig. 5 C and D shows the psychometric (2.8 Hz) and neurometric (4.41 Hz) thresholds; the psychometric/neurometric threshold ratio = 0.65. Fig. 5E indicates that the psychometric and neurometric threshold ratios computed during the discrimination of periodic (1.14 ± 0.78; open bars) and aperiodic (1.04 ± 0.75; black bars) stimuli are quite similar. As in the periodic condition, a psychophysical observer could exploit firing rate for frequency discrimination of aperiodic stimuli.
Figure 5.
Firing rate modulation of an area 1 neuron during the discrimination of aperiodic stimuli. The same format as Fig. 3A, but both base (f1) and comparison (f2) frequencies (mean frequencies) lack periodicity. (A) Raster plots. (B) Mean firing rate (± SD) as a function of the stimulus frequency. (C) Relationship between psychometric and neurometric functions. (D) Psychometric and neurometric functions. (E) Threshold ratios (psychometric/neurometric thresholds) for each neuron during the discrimination of periodic stimulus frequencies (open bars). Black bars represent the threshold ratios between psychometric and neurometric thresholds during the discrimination of aperiodic stimulus frequencies.
We have shown two clearly different response patterns elicited by the flutter stimuli in the QA neuronal population. Are these modulations restricted to the flutter range? To respond to this question, in separate recordings from QA neurons (n = 25), using a broad range of vibration frequencies at amplitude detection thresholds (2–200 Hz), we have determined that those neurons that reproduce the periodicity (n = 15) or that vary average firing rate (n = 10) can be approximately described as that of a low-pass linear filter with a cutoff frequency at ≈60 Hz. This was determined by the use of a Bode plot (17, 18). QA neurons that are periodically entrained by the stimulus frequencies showed the nonlinearities (rectification and phase-locking at high frequencies in the flutter range, 20–50 Hz) reported in the QA primary afferents (19, 20). In particular, phase-locked responses would limit firing rate modulation.
Discussion
We sought to identify which component(s) of the evoked activity of S1 neurons is/are sufficient for frequency discrimination. We found that the periodic mechanical stimulus frequency can be represented both in the periodicity and in the firing rate responses to varying degrees across the QA neuronal population of S1. The analysis revealed that neurometric thresholds computed by using the periodic spike intervals are far lower than the psychometric thresholds. This is not the case for the group of QA neurons that modulate their firing rate as a function of the periodic or aperiodic stimulus frequency, and so permit calculation of a neurometric threshold based on firing rate that is closely similar to the psychometric thresholds. The goal of computing such neurometric functions was not only to reveal the relationship between the neuronal responses of S1 to the mechanical stimulus, but also to discern whether these neural signals account for the psychophysical behavior.
The conclusion previously found in the literature, that frequency discrimination is based on periodicity, came from the observation that a small number of studied QA neurons from S1 reproduce in their activity the periodicity of the mechanical stimulus frequency, and also from the fact that these neurons did not have average firing rates that were modulated by the stimulus frequency (7). However, the study that reached this conclusion only determined the relationship between the neuronal responses to the mechanical stimulus frequencies; no attempt was made to quantify the neurometric thresholds based on periodicity and to compare these to the psychophysical thresholds. Our analysis shows that neurometric thresholds using the periodicity are far lower than the psychometric thresholds. What is then the functional meaning of this neural signal? One possible role is that they simply represent the temporal structure of the stimulus and that monkeys do not use this exquisite representation for frequency discrimination. Consistent with this idea, we found QA neurons in S1 whose firing rates are modulated by the stimulus frequencies, and their neurometric thresholds based on firing rate are closely similar to the monkeys' psychophysical thresholds.
Because monkeys discriminate between aperiodic stimuli with psychophysical thresholds similar to those obtained during discrimination of periodic stimuli, and because of the close similarity we found (during discrimination of periodic stimuli) between firing-rate-based neurometric and psychometric thresholds, we are tempted to suggest that firing rate, and not periodicity, could be the neural signal at the level of S1 that a psychophysical observer (e.g., processes central to S1) could use for stimulus frequency discrimination. This fits quite well with the criteria discussed by Parker and Newsome to validate a neural code for sensory discrimination (2).
Observation of a neuronal correlate does not prove that the neuronal response is sufficient for frequency discrimination. However, microstimulation experiments have shown that the relationship between these neuronal responses and the animal's behavior are not simple coincidences (10, 11). Monkeys are able to discriminate among periodic and aperiodic stimulus frequencies either delivered to the fingertips or artificially injected into a cluster of QA neurons (10, 11). These findings indicate that the evoked neuronal activity in the QA circuit of S1 is necessary for frequency discrimination whereas periodicity seems unnecessary to solve the task. Interestingly, it has been suggested that discrimination in the range of 50–300 Hz is made by using a mean firing rate code from a neuronal population of S1 linked to the Pacinian (PC) mechanoreceptors (4, 5, 7). Additionally, direct microstimulation of the QA primary afferents produced flutter sensations of frequencies that were perceived to increase with the evoked firing rate (21). According to our results, a simple rate code could suffice for frequency discrimination along the entire range of the flutter-vibration (5–300 Hz). In support of this, we have found that most neurons of the second somatosensory cortex—a cortical area in which S1 neurons project (22–25)—reflect the discrimination process of flutter stimuli in their firing rate, but not in periodicity (data not shown). In recordings from the prefrontal cortex during the discrimination task, we found neurons encoding the base stimulus frequency in their firing rate during the delay interstimulus period (26). Thus, what we have observed in these two cortical areas, including a population of QA neurons of the S1, is a firing rate signal that could suffice to encode the stimulus frequency. This neural representation is closely associated with the psychophysical demands of this sensory discrimination task.
Our results also suggest that QA neurons of S1, which are classified according to their capacity to react to a slight mechanical indentation applied to the center of their receptive fields, may in fact be composed of two subpopulations, each of which behaves differently in response to a periodic mechanical stimulus. These two subpopulations might be arranged in hierarchical fashion: QA neurons that respond periodically might be closer to the input stimulus, and those that modulate their firing rate might integrate the responses of the periodic neurons and transform them into a rate code. Such last order neurons of the QA circuit could distribute the neural representation to those structures anatomically linked to S1, to solve the sensory discrimination task. Further studies will be needed to test whether this is so.
The slowly adapting (SA) and the PC neurons of S1 are also activated in the flutter discrimination task. Do these neurons contribute to the frequency discrimination task? To address this issue, we studied 77 SA neurons of S1 during the discrimination task. Half of the SA neurons responded with periodic spike intervals at the input stimulus frequency (7), but we found that none of them modulated their firing rate. Neurometric thresholds based on periodicity for these neurons were lower than psychophysical thresholds (the psychometric/neurometric threshold ratio = 4.48 ± 2.39). However, microstimulation studies have indicated that, although SA neurons respond in a stimulus-dependent manner, they do not participate in the perceptual encoding of flutter frequencies (10, 11). Neurons associated with PC type properties were recorded only extremely rarely (a total of four PC neurons). None of these showed periodic responses; they simply responded by increasing the firing rate during the stimulus periods, but were not frequency modulated. We do not know whether this is attributable to a training effect. Thus, the suprathreshold stimuli used in our task activated the SA and PC neurons of S1, but only the QA neurons seem to provide meaningful information for flutter discrimination (present results; see refs. 10 and 11). It is tempting to compare these results with the proposed peripheral neural coding of vibrotactile stimuli. Psychophysical studies in humans and recording of peripheral fibers at detection thresholds have revealed that the response properties of these fibers contribute to the range of frequencies between 2 and 300 Hz (4, 27, 28). These results are the basis for understanding peripheral neural coding of vibrotactile stimuli at detection thresholds; however, they not necessarily apply to understanding the cortical neural coding of flutter discrimination.
To conclude, the periodic mechanical stimulus frequency can be represented both in the periodicity and in the firing rate responses to varying degrees across the QA neuronal population of S1. Such a double representation allows asking which of the two signals is actually used by the animals for discrimination. To our knowledge, the present study is the first combined neurophysiological/psychophysical study that directly addressed the question of which neural signal in S1 is more likely to be used for sensory discrimination. Finally, it is tempting to compare our results with similar observations in other sensory modalities. Unfortunately, comparison with other sensory modalities at this stage is made difficult by the fact that most combined neurophysiological/psychophysical studies have recorded from structures central to primary sensory cortices (2, 29).
Acknowledgments
We thank C. Brody, W. T. Newsome, E. Salinas, and M. Shadlen for invaluable comments and discussions. We appreciate the technical assistance of Luis Lemus, Federico Jandete, and Sergio Méndez. Research by R.R. was supported in part by an International Research Scholars Award from the Howard Hughes Medical Institute, Dirección General de Asuntos del Personal Académico/UNAM, and Consejo Nacional de Ciencia y Tecnología.
Abbreviations
- QA
quickly adapting
- SA
slowly adapting
- ROC
receiver operating characteristic
- PC
Pacinian
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120018597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120018597
References
- 1.Shadlen M N, Newsome W T. Curr Opin Neurobiol. 1994;4:569–579. doi: 10.1016/0959-4388(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 2.Parker A J, Newsome W T. Annu Rev Neurosci. 1998;21:227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- 3.Romo R, Salinas E. Curr Opin Neurobiol. 1999;9:487–493. doi: 10.1016/S0959-4388(99)80073-7. [DOI] [PubMed] [Google Scholar]
- 4.Talbot W H, Darian-Smith I, Kornhuber H H, Mountcastle V B. J Neurophysiol. 1968;31:301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- 5.Mountcastle V B, Talbot W H, Sakata H, Hyvarinen J. J Neurophysiol. 1969;32:453–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- 6.LaMotte R H, Mountcastle V B. J Neurophysiol. 1975;38:539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- 7.Mountcastle V B, Steinmetz M A, Romo R. J Neurosci. 1990;10:3032–3044. doi: 10.1523/JNEUROSCI.10-09-03032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Recanzone G H, Merzenich M M, Schreiner C E. J Neurophysiol. 1992;67:1071–1091. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- 9.Hernández A, Salinas E, García R, Romo R. J Neurosci. 1997;17:6391–6400. doi: 10.1523/JNEUROSCI.17-16-06391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romo R, Hernández A, Zainos A, Salinas E. Nature (London) 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- 11.Romo R, Hernández A, Zainos A, Brody C D, Lemus L. Neuron. 2000;26:273–278. doi: 10.1016/s0896-6273(00)81156-3. [DOI] [PubMed] [Google Scholar]
- 12.Mountcastle V B, Reitboeck H J, Poggio G F, Steinmetz M M. J Neurosci Methods. 1991;36:77–84. doi: 10.1016/0165-0270(91)90140-u. [DOI] [PubMed] [Google Scholar]
- 13.Press W, Teukolsky S A, Vettering W T, Fannery B P. Numerical Recipes in C. Cambridge, U.K.: Cambridge Univ. Press; 1992. [Google Scholar]
- 14.Siegel S, Castellan N J. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw–Hill; 1988. [Google Scholar]
- 15.Ross S M. Introduction to Probability for Scientists and Engineers. New York: Wiley; 1987. [Google Scholar]
- 16.Green D M, Sweets J A. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- 17.D'Azzo J J, Houpis C. Feedback Control System Analysis and Synthesis. Tokyo: McGraw–Hill; 1966. [Google Scholar]
- 18.Ogata K. Modern Control Engineering. Englewood Cliffs, New Jersey: Prentice–Hall; 1990. [Google Scholar]
- 19.Freeman A W, Johnson K O. J Physiol (London) 1982;323:21–41. doi: 10.1113/jphysiol.1982.sp014059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loof F J, Baltensperger C M. IEEE Trans Biomed Eng. 1990;37:565–573. doi: 10.1109/10.55660. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa J, Torebjörk E. J Physiol (London) 1983;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pons T P, Garraghty P E, Friedman D P, Mishkin M. Science. 1987;237:417–420. doi: 10.1126/science.3603028. [DOI] [PubMed] [Google Scholar]
- 23.Pons T P, Garraghty P E, Mishkin M. J Neurophysiol. 1992;68:518–527. doi: 10.1152/jn.1992.68.2.518. [DOI] [PubMed] [Google Scholar]
- 24.Burton H, Fabri M, Alloway K. J Comp Neurol. 1995;355:539–562. doi: 10.1002/cne.903550405. [DOI] [PubMed] [Google Scholar]
- 25.Krubitzer L A, Clarey J, Tweedale R, Elston G, Calford M. J Neurosci. 1995;15:3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romo R, Brody C D, Hernández A, Lemus L. Nature (London) 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 27.Verrillo R T. J Acoust Soc Am. 1985;77:225–232. doi: 10.1121/1.392263. [DOI] [PubMed] [Google Scholar]
- 28.Bolanowski S J, Gescheider G A, Verrillo R T, Checkosky C M. J Acoust Soc Am. 1988;84:1680–1694. doi: 10.1121/1.397184. [DOI] [PubMed] [Google Scholar]
- 29.Vogels R, Orban G A. J Neurosci. 1990;10:3543–3558. doi: 10.1523/JNEUROSCI.10-11-03543.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]