Abstract
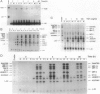
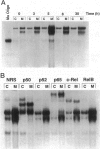
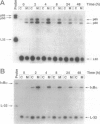
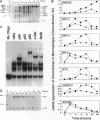
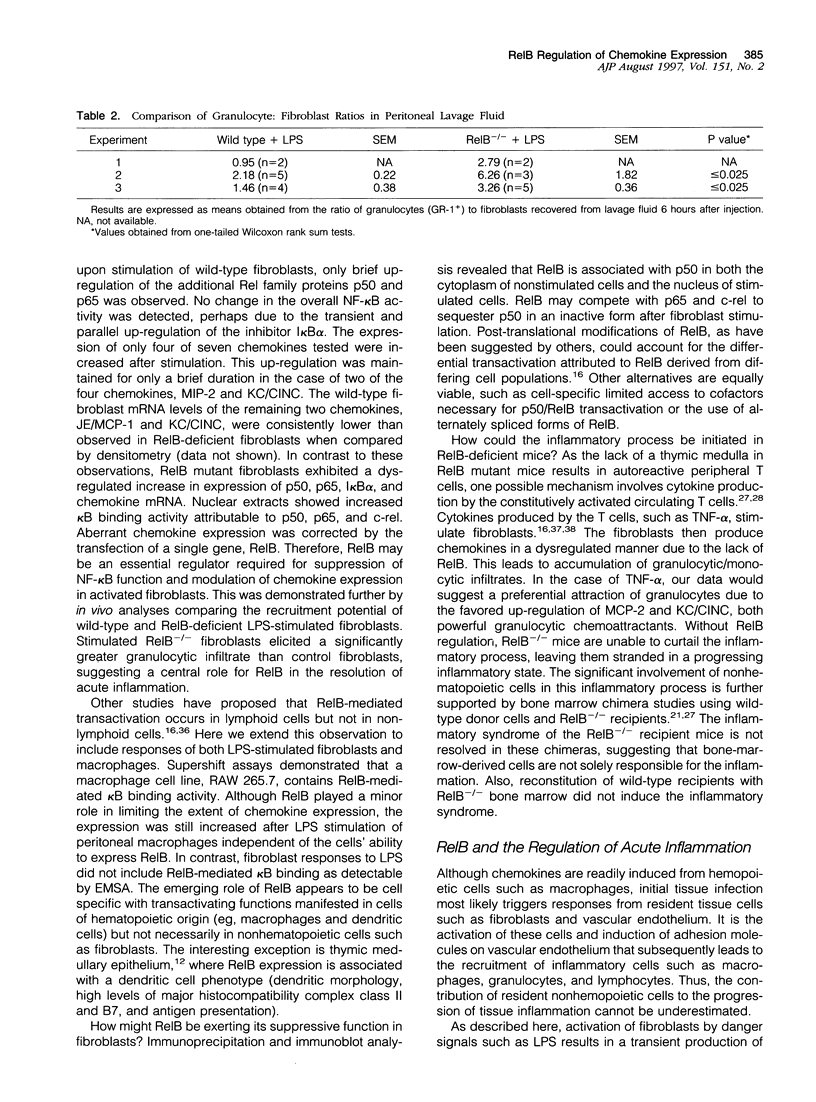
The resolution of acute inflammation is incompletely understood but presumably requires the elimination of both inflammatory cells and production of inflammatory cytokines. In the case of recruited bone-marrow-derived inflammatory cells such as granulocytes and macrophages, their short life span helps eliminate these cells and the cytokines they produce. By contrast, resident permanent cells such as fibroblasts require other mechanisms to stop the production of chemokines generated in response to inflammatory triggers such as lipopolysaccharide. Here we demonstrate that RelB is an important regulator of chemokine expression in fibroblasts, thereby playing a key role in the resolution of acute inflammation. Activation of normal fibroblasts by lipopolysaccharide induced a transient production of chemokines, closely followed by induction of RelB expression. However, stimulated RelB-/- fibroblasts exhibited dramatic persistent induction of seven chemokines (RANTES, MIP-1 alpha, MIP-1 beta, MIP-2, IP-10, JE/MCP-1, and KC/CINC). The persistent overexpression of chemokines correlated with increased NF- kappa B binding as well as with increased p50, p65/RelA, and I kappa B alpha expression. Transfection of RelB cDNA into RelB-deficient fibroblasts reversed the lipopolysaccharide-induced chemokine overexpression. In vivo, activated RelB-/- fibroblasts dramatically increased recruitment of granulocytes into tissues. In view of the apparent role of RelB in the resolution of acute inflammation in tissues and previous work showing a requirement for RelB in the initiation of immune responses through the differentiation of antigen-presenting cells, RelB may be an important factor regulating the transition from innate to adaptive immunity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. NF-kappa B: ten years after. Cell. 1996 Oct 4;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Sha W. C., Bronson R. T., Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995 Nov 15;9(22):2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Sha W. C., Bronson R. T., Ghosh S., Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995 Jul 13;376(6536):167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Bombara M. P., Webb D. L., Conrad P., Marlor C. W., Sarr T., Ranges G. E., Aune T. M., Greve J. M., Blue M. L. Cell contact between T cells and synovial fibroblasts causes induction of adhesion molecules and cytokines. J Leukoc Biol. 1993 Nov;54(5):399–406. doi: 10.1002/jlb.54.5.399. [DOI] [PubMed] [Google Scholar]
- Bours V., Azarenko V., Dejardin E., Siebenlist U. Human RelB (I-Rel) functions as a kappa B site-dependent transactivating member of the family of Rel-related proteins. Oncogene. 1994 Jun;9(6):1699–1702. [PubMed] [Google Scholar]
- Burkly L., Hession C., Ogata L., Reilly C., Marconi L. A., Olson D., Tizard R., Cate R., Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995 Feb 9;373(6514):531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Zbrzezna V., Polyakova J., Pogo A. O., Hesselgesser J., Horuk R. Expression of the Duffy antigen in K562 cells. Evidence that it is the human erythrocyte chemokine receptor. J Biol Chem. 1994 Mar 18;269(11):7835–7838. [PubMed] [Google Scholar]
- Cheng Q., Cant C. A., Moll T., Hofer-Warbinek R., Wagner E., Birnstiel M. L., Bach F. H., de Martin R. NK-kappa B subunit-specific regulation of the I kappa B alpha promoter. J Biol Chem. 1994 May 6;269(18):13551–13557. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeKoning J., DiMolfetto L., Reilly C., Wei Q., Havran W. L., Lo D. Thymic cortical epithelium is sufficient for the development of mature T cells in relB-deficient mice. J Immunol. 1997 Mar 15;158(6):2558–2566. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanski P., Ryseck R. P., Bravo R. Both N- and C-terminal domains of RelB are required for full transactivation: role of the N-terminal leucine zipper-like motif. Mol Cell Biol. 1993 Mar;13(3):1572–1582. doi: 10.1128/mcb.13.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanski P., Ryseck R. P., Bravo R. Specific inhibition of RelB/p52 transcriptional activity by the C-terminal domain of p100. Oncogene. 1995 Mar 2;10(5):1003–1007. [PubMed] [Google Scholar]
- Feng L., Xia Y., Kreisberg J. I., Wilson C. B. Interleukin-1 alpha stimulates KC synthesis in rat mesangial cells: glucocorticoids inhibit KC induction by IL-1. Am J Physiol. 1994 May;266(5 Pt 2):F713–F722. doi: 10.1152/ajprenal.1994.266.5.F713. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Randolph G. J. Chemokines and tissue injury. Am J Pathol. 1995 Jun;146(6):1287–1301. [PMC free article] [PubMed] [Google Scholar]
- Geiser T., Dewald B., Ehrengruber M. U., Clark-Lewis I., Baggiolini M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J Biol Chem. 1993 Jul 25;268(21):15419–15424. [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Ito C. Y., Kazantsev A. G., Baldwin A. S., Jr Three NF-kappa B sites in the I kappa B-alpha promoter are required for induction of gene expression by TNF alpha. Nucleic Acids Res. 1994 Sep 11;22(18):3787–3792. doi: 10.1093/nar/22.18.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer T. M., DeKoning J., Markowitz J. S., Lo D., Glimcher L. H. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996 Sep 5;383(6595):81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- Lernbecher T., Kistler B., Wirth T. Two distinct mechanisms contribute to the constitutive activation of RelB in lymphoid cells. EMBO J. 1994 Sep 1;13(17):4060–4069. doi: 10.1002/j.1460-2075.1994.tb06723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernbecher T., Müller U., Wirth T. Distinct NF-kappa B/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature. 1993 Oct 21;365(6448):767–770. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- Lo D., Quill H., Burkly L., Scott B., Palmiter R. D., Brinster R. L. A recessive defect in lymphocyte or granulocyte function caused by an integrated transgene. Am J Pathol. 1992 Nov;141(5):1237–1246. [PMC free article] [PubMed] [Google Scholar]
- Mauviel A., Reitamo S., Remitz A., Lapière J. C., Ceska M., Baggiolini M., Walz A., Evans C. H., Uitto J. Leukoregulin, a T cell-derived cytokine, induces IL-8 gene expression and secretion in human skin fibroblasts. Demonstration and secretion in human skin fibroblasts. Demonstration of enhanced NF-kappa B binding and NF-kappa B-driven promoter activity. J Immunol. 1992 Nov 1;149(9):2969–2976. [PubMed] [Google Scholar]
- Rot A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur J Immunol. 1993 Jan;23(1):303–306. doi: 10.1002/eji.1830230150. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Bull P., Takamiya M., Bours V., Siebenlist U., Dobrzanski P., Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992 Feb;12(2):674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Bacon K. B. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994 Dec;6(6):865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Ganchi P. A., Ballard D. W., Greene W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993 Mar 26;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Sylvester I., Suffredini A. F., Boujoukos A. J., Martich G. D., Danner R. L., Yoshimura T., Leonard E. J. Neutrophil attractant protein-1 and monocyte chemoattractant protein-1 in human serum. Effects of intravenous lipopolysaccharide on free attractants, specific IgG autoantibodies and immune complexes. J Immunol. 1993 Sep 15;151(6):3292–3298. [PubMed] [Google Scholar]
- Vaddi K., Newton R. C. Regulation of monocyte integrin expression by beta-family chemokines. J Immunol. 1994 Nov 15;153(10):4721–4732. [PubMed] [Google Scholar]
- Walz A., Meloni F., Clark-Lewis I., von Tscharner V., Baggiolini M. [Ca2+]i changes and respiratory burst in human neutrophils and monocytes induced by NAP-1/interleukin-8, NAP-2, and gro/MGSA. J Leukoc Biol. 1991 Sep;50(3):279–286. doi: 10.1002/jlb.50.3.279. [DOI] [PubMed] [Google Scholar]
- Weih F., Carrasco D., Durham S. K., Barton D. S., Rizzo C. A., Ryseck R. P., Lira S. A., Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995 Jan 27;80(2):331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Weih F., Lira S. A., Bravo R. Overexpression of RelB in transgenic mice does not affect I kappa B alpha levels: differential regulation of RelA and RelB by the inhibitor protein. Oncogene. 1996 Jan 18;12(2):445–449. [PubMed] [Google Scholar]
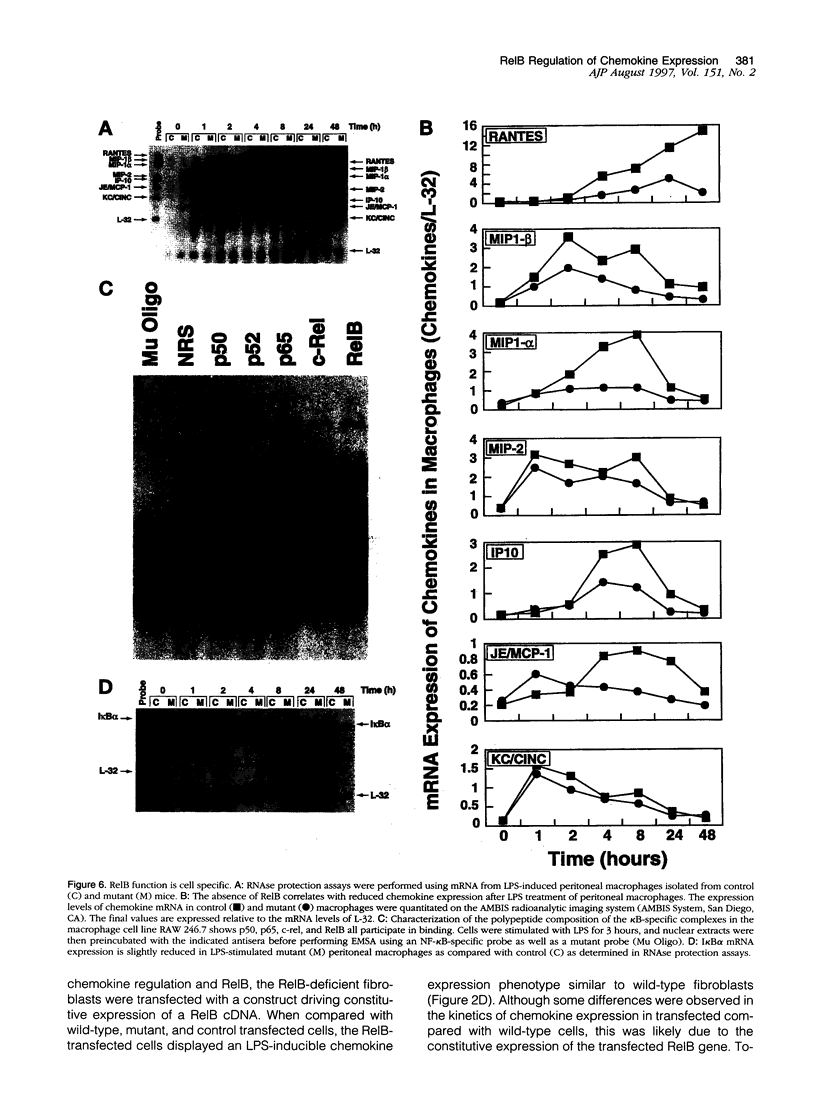
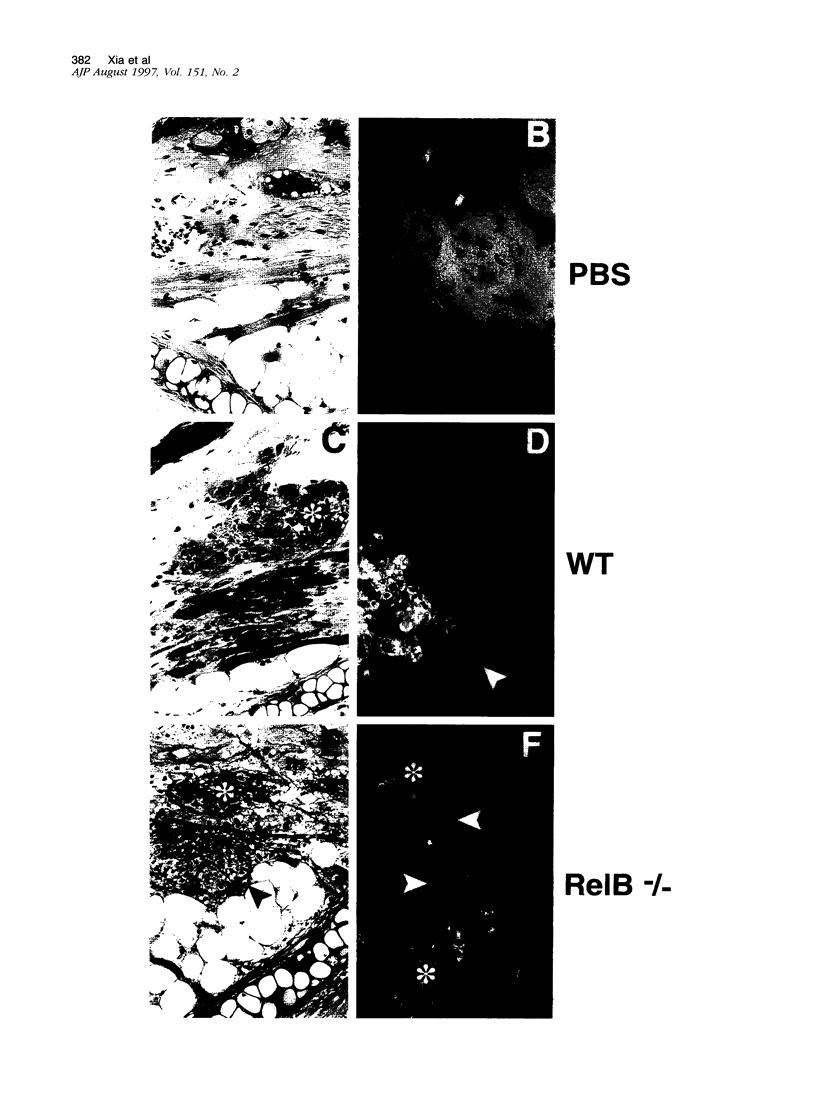
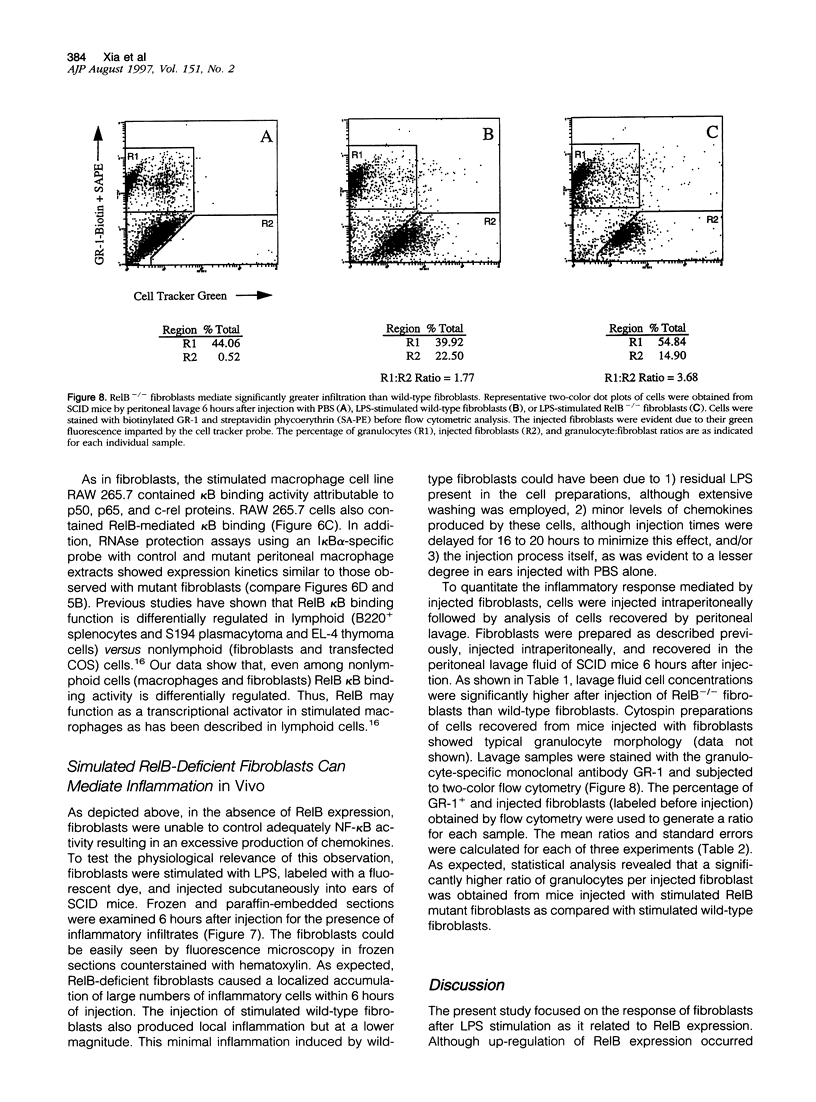
- Widmer U., Manogue K. R., Cerami A., Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J Immunol. 1993 Jun 1;150(11):4996–5012. [PubMed] [Google Scholar]