Abstract
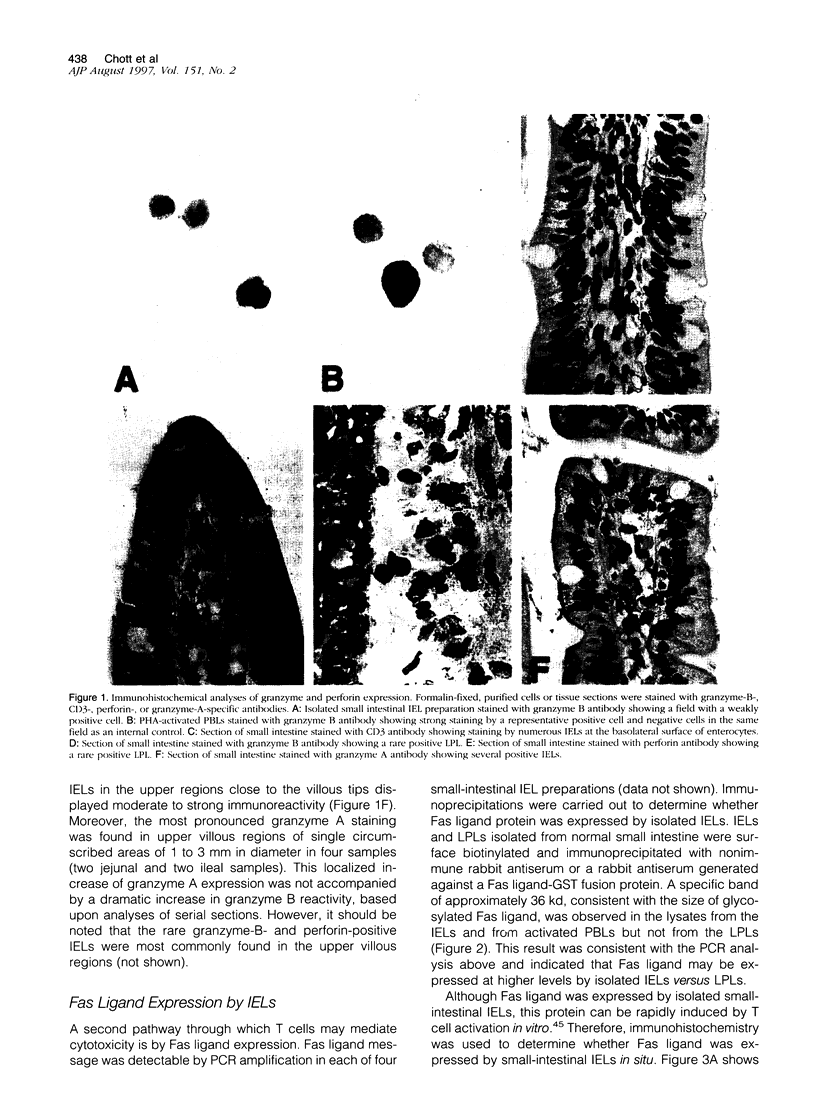
Human small intestine contains a very large population of intraepithelial T lymphocytes (IELs) that are oligoclonal, appear functionally to be cytolytic T cells, and may contribute to the normal and pathological turnover of intestinal epithelial cells. This report addresses the cytolytic function of IELs in normal small intestine by examining their expression of molecules that carry out cell-mediated cytolysis. Immunohistochemical analyses of granzyme B, perforin, Fas ligand, and tumor necrosis factor-alpha demonstrated these proteins were not expressed by small intestinal IELs in situ. These proteins also were not expressed by colonic IELs or by lamina propria lymphocytes in the small or large intestine. Granzyme A, however, was expressed by a large fraction of IELs. In contrast to these in situ results, isolated and in vitro activated IELs were shown to express effector proteins consistent with cytolytic T cells, including granzyme B, Fas ligand, tumor necrosis factor-alpha, and interferon-gamma. These results are most consistent with the vast majority of IELs in normal human small intestine being resting cytolytic T cells and suggest that these cells do not contribute to the apoptotic cell death of epithelial cells in normal intestine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Tough T. W., Davis-Smith T., Braddy S., Falk B., Schooley K. A., Goodwin R. G., Smith C. A., Ramsdell F., Lynch D. H. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995 Jan 1;181(1):71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. P., Ebert E. C., Blumenthal R. L., McDermott F. V., Wucherpfennig K. W., Landau S. B., Blumberg R. S. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991 Sep 20;253(5026):1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- Beil W. J., Weller P. F., Peppercorn M. A., Galli S. J., Dvorak A. M. Ultrastructural immunogold localization of subcellular sites of TNF-alpha in colonic Crohn's disease. J Leukoc Biol. 1995 Sep;58(3):284–298. doi: 10.1002/jlb.58.3.284. [DOI] [PubMed] [Google Scholar]
- Blumberg R. S., Yockey C. E., Gross G. G., Ebert E. C., Balk S. P. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilize multiple V beta T cell receptor genes. J Immunol. 1993 Jun 1;150(11):5144–5153. [PubMed] [Google Scholar]
- Boismenu R., Havran W. L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994 Nov 18;266(5188):1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Bombí J. A., Nadal A., Carreras E., Ramírez J., Muñoz J., Rozman C., Cardesa A. Assessment of histopathologic changes in the colonic biopsy in acute graft-versus-host disease. Am J Clin Pathol. 1995 Jun;103(6):690–695. doi: 10.1093/ajcp/103.6.690. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Bosnes V., Halstensen T. S., Scott H., Sollid L. M., Valnes K. N. T lymphocytes in human gut epithelium preferentially express the alpha/beta antigen receptor and are often CD45/UCHL1-positive. Scand J Immunol. 1989 Jul;30(1):123–128. doi: 10.1111/j.1365-3083.1989.tb01196.x. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Brousse N., Lisowska-Grospierre B., Guy-Grand D., Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987 Sep;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Chott A., Probert C. S., Gross G. G., Blumberg R. S., Balk S. P. A common TCR beta-chain expressed by CD8+ intestinal mucosa T cells in ulcerative colitis. J Immunol. 1996 Apr 15;156(8):3024–3035. [PubMed] [Google Scholar]
- Chowers Y., Holtmeier W., Harwood J., Morzycka-Wroblewska E., Kagnoff M. F. The V delta 1 T cell receptor repertoire in human small intestine and colon. J Exp Med. 1994 Jul 1;180(1):183–190. doi: 10.1084/jem.180.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria R., Boirivant M., Cifone M. G., Roncaioli P., Hahne M., Tschopp J., Pallone F., Santoni A., Testi R. Functional expression of Fas and Fas ligand on human gut lamina propria T lymphocytes. A potential role for the acidic sphingomyelinase pathway in normal immunoregulation. J Clin Invest. 1996 Jan 15;97(2):316–322. doi: 10.1172/JCI118418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deusch K., Lüling F., Reich K., Classen M., Wagner H., Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur J Immunol. 1991 Apr;21(4):1053–1059. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- Ebert E. C. Proliferative responses of human intraepithelial lymphocytes to various T-cell stimuli. Gastroenterology. 1989 Dec;97(6):1372–1381. doi: 10.1016/0016-5085(89)90379-x. [DOI] [PubMed] [Google Scholar]
- Ebert E. C., Roberts A. I., Brolin R. E., Raska K. Examination of the low proliferative capacity of human jejunal intraepithelial lymphocytes. Clin Exp Immunol. 1986 Jul;65(1):148–157. [PMC free article] [PubMed] [Google Scholar]
- Ebnet K., Hausmann M., Lehmann-Grube F., Müllbacher A., Kopf M., Lamers M., Simon M. M. Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 1995 Sep 1;14(17):4230–4239. doi: 10.1002/j.1460-2075.1995.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramzinski R. A., Adams E., Gross J. A., Goodman T. G., Allison J. P., Lefrançois L. T cell receptor-triggered activation of intraepithelial lymphocytes in vitro. Int Immunol. 1993 Feb;5(2):145–153. doi: 10.1093/intimm/5.2.145. [DOI] [PubMed] [Google Scholar]
- Gross G. G., Schwartz V. L., Stevens C., Ebert E. C., Blumberg R. S., Balk S. P. Distribution of dominant T cell receptor beta chains in human intestinal mucosa. J Exp Med. 1994 Oct 1;180(4):1337–1344. doi: 10.1084/jem.180.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Malassis-Seris M., Briottet C., Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991 Jun 1;173(6):1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994 Mar 25;76(6):977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Jarry A., Cerf-Bensussan N., Brousse N., Selz F., Guy-Grand D. Subsets of CD3+ (T cell receptor alpha/beta or gamma/delta) and CD3- lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur J Immunol. 1990 May;20(5):1097–1103. doi: 10.1002/eji.1830200523. [DOI] [PubMed] [Google Scholar]
- Kojima H., Shinohara N., Hanaoka S., Someya-Shirota Y., Takagaki Y., Ohno H., Saito T., Katayama T., Yagita H., Okumura K. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994 Aug;1(5):357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Kotler D. P., Weaver S. C., Terzakis J. A. Ultrastructural features of epithelial cell degeneration in rectal crypts of patients with AIDS. Am J Surg Pathol. 1986 Aug;10(8):531–538. doi: 10.1097/00000478-198608000-00002. [DOI] [PubMed] [Google Scholar]
- Kummer J. A., Kamp A. M., van Katwijk M., Brakenhoff J. P., Radosević K., van Leeuwen A. M., Borst J., Verweij C. L., Hack C. E. Production and characterization of monoclonal antibodies raised against recombinant human granzymes A and B and showing cross reactions with the natural proteins. J Immunol Methods. 1993 Jul 6;163(1):77–83. doi: 10.1016/0022-1759(93)90241-x. [DOI] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Zinkernagel R. M., Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994 Jul 22;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Lee F. D. Importance of apoptosis in the histopathology of drug related lesions in the large intestine. J Clin Pathol. 1993 Feb;46(2):118–122. doi: 10.1136/jcp.46.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L., Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989 Mar 31;243(4899):1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- Leithäuser F., Dhein J., Mechtersheimer G., Koretz K., Brüderlein S., Henne C., Schmidt A., Debatin K. M., Krammer P. H., Möller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993 Oct;69(4):415–429. [PubMed] [Google Scholar]
- Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994 Aug 25;370(6491):650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Lundqvist C., Baranov V., Hammarström S., Athlin L., Hammarström M. L. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995 Sep;7(9):1473–1487. doi: 10.1093/intimm/7.9.1473. [DOI] [PubMed] [Google Scholar]
- Lundqvist C., Melgar S., Yeung M. M., Hammarström S., Hammarström M. L. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J Immunol. 1996 Sep 1;157(5):1926–1934. [PubMed] [Google Scholar]
- Murch S. H., Braegger C. P., Walker-Smith J. A., MacDonald T. T. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993 Dec;34(12):1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller P., Walczak H., Reidl S., Sträter J., Krammer P. H. Paneth cells express high levels of CD95 ligand transcripts: a unique property among gastrointestinal epithelia. Am J Pathol. 1996 Jul;149(1):9–13. [PMC free article] [PubMed] [Google Scholar]
- Müllbacher A., Ebnet K., Blanden R. V., Hla R. T., Stehle T., Museteanu C., Simon M. M. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber G., Vogelsang H., Stolte M., Muthenthaler S., Kummer J. A., Kummer A. J., Radaszkiewicz T. Evidence that intestinal intraepithelial lymphocytes are activated cytotoxic T cells in celiac disease but not in giardiasis. Am J Pathol. 1996 May;148(5):1351–1357. [PMC free article] [PubMed] [Google Scholar]
- Pirzer U. C., Schürmann G., Post S., Betzler M., Meuer S. C. Differential responsiveness to CD3-Ti vs. CD2-dependent activation of human intestinal T lymphocytes. Eur J Immunol. 1990 Oct;20(10):2339–2342. doi: 10.1002/eji.1830201025. [DOI] [PubMed] [Google Scholar]
- Ratner A., Clark W. R. Role of TNF-alpha in CD8+ cytotoxic T lymphocyte-mediated lysis. J Immunol. 1993 May 15;150(10):4303–4314. [PubMed] [Google Scholar]
- Russell G. J., Nagler-Anderson C., Anderson P., Bhan A. K. Cytotoxic potential of intraepithelial lymphocytes (IELs). Presence of TIA-1, the cytolytic granule-associated protein, in human IELs in normal and diseased intestine. Am J Pathol. 1993 Aug;143(2):350–354. [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Jewell D. P. Immunohistological characterisation of intraepithelial lymphocytes of the human gastrointestinal tract. Gut. 1981 Mar;22(3):169–176. doi: 10.1136/gut.22.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara T., Sato N., Waguri S., Iwanaga T., Nakahara A., Fukutomi H., Uchiyama Y. The fate of effete epithelial cells at the villus tips of the human small intestine. Arch Histol Cytol. 1995 Jun;58(2):205–219. doi: 10.1679/aohc.58.205. [DOI] [PubMed] [Google Scholar]
- Sträter J., Koretz K., Günthert A. R., Möller P. In situ detection of enterocytic apoptosis in normal colonic mucosa and in familial adenomatous polyposis. Gut. 1995 Dec;37(6):819–825. doi: 10.1136/gut.37.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Sydora B. C., Mixter P. F., Holcombe H. R., Eghtesady P., Williams K., Amaral M. C., Nel A., Kronenberg M. Intestinal intraepithelial lymphocytes are activated and cytolytic but do not proliferate as well as other T cells in response to mitogenic signals. J Immunol. 1993 Mar 15;150(6):2179–2191. [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Inazawa J., Abe T., Suda T., Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994 Oct;6(10):1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- Tan X., Hsueh W., Gonzalez-Crussi F. Cellular localization of tumor necrosis factor (TNF)-alpha transcripts in normal bowel and in necrotizing enterocolitis. TNF gene expression by Paneth cells, intestinal eosinophils, and macrophages. Am J Pathol. 1993 Jun;142(6):1858–1865. [PMC free article] [PubMed] [Google Scholar]
- Taunk J., Roberts A. I., Ebert E. C. Spontaneous cytotoxicity of human intraepithelial lymphocytes against epithelial cell tumors. Gastroenterology. 1992 Jan;102(1):69–75. doi: 10.1016/0016-5085(92)91785-3. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Smart C. J., Oakes D. J., Howdle P. D., Malizia G., Campana D., Boylston A. W. Expression of T-cell receptors TcR1 (gamma/delta) and TcR2 (alpha/beta) in the human intestinal mucosa. Immunology. 1989 Sep;68(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Van Kerckhove C., Russell G. J., Deusch K., Reich K., Bhan A. K., DerSimonian H., Brenner M. B. Oligoclonality of human intestinal intraepithelial T cells. J Exp Med. 1992 Jan 1;175(1):57–63. doi: 10.1084/jem.175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney J. L., Kilshaw P. J., MacDonald T. T. Cytotoxic alpha/beta+ and gamma/delta+ T cells in murine intestinal epithelium. Eur J Immunol. 1990 Jul;20(7):1623–1626. doi: 10.1002/eji.1830200734. [DOI] [PubMed] [Google Scholar]
- Walsh C. M., Matloubian M., Liu C. C., Ueda R., Kurahara C. G., Christensen J. L., Huang M. T., Young J. D., Ahmed R., Clark W. R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Ueno Y., Yajima T., Iwao Y., Tsuchiya M., Ishikawa H., Aiso S., Hibi T., Ishii H. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995 Jun;95(6):2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]