Abstract
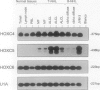
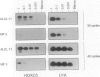
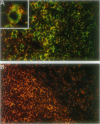
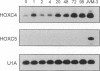
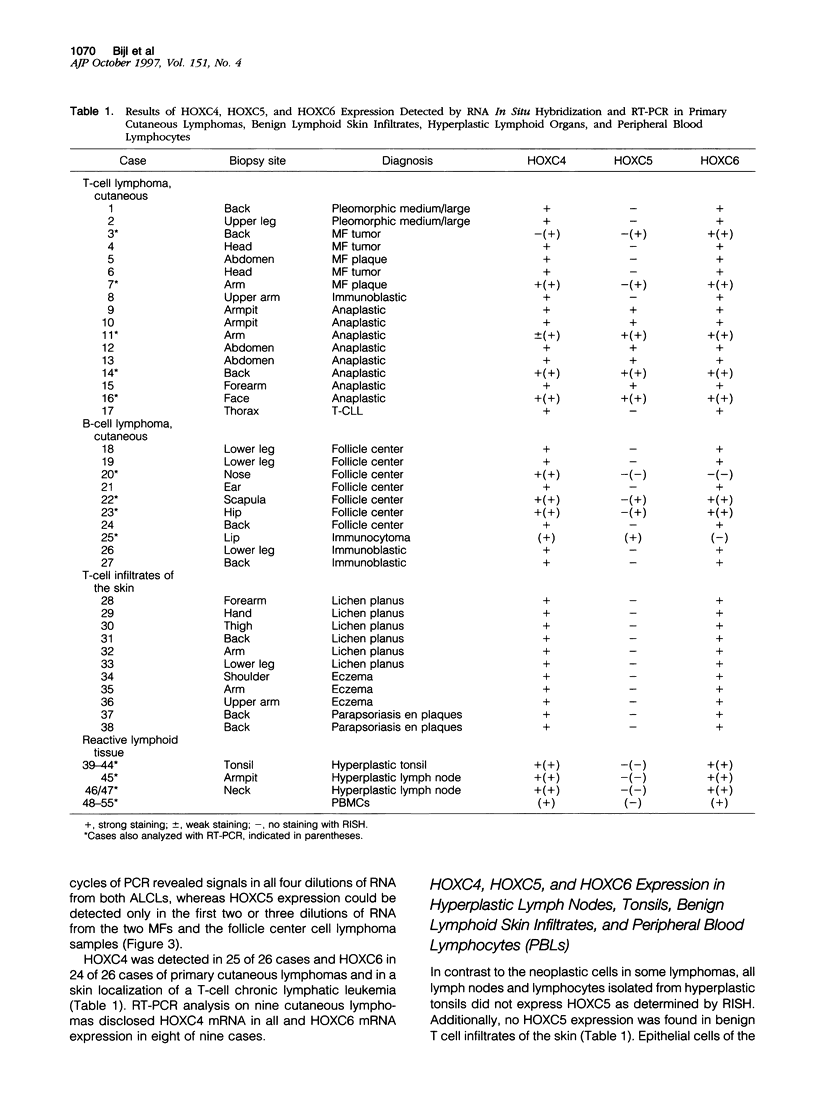
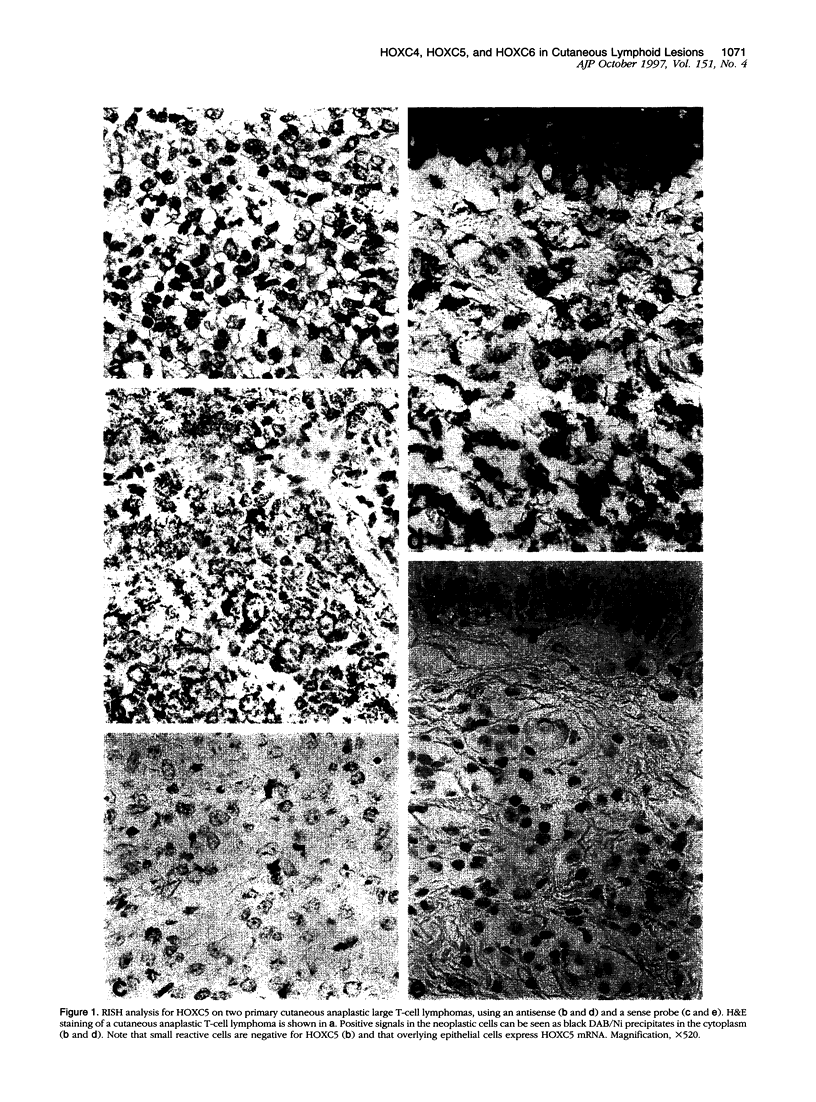
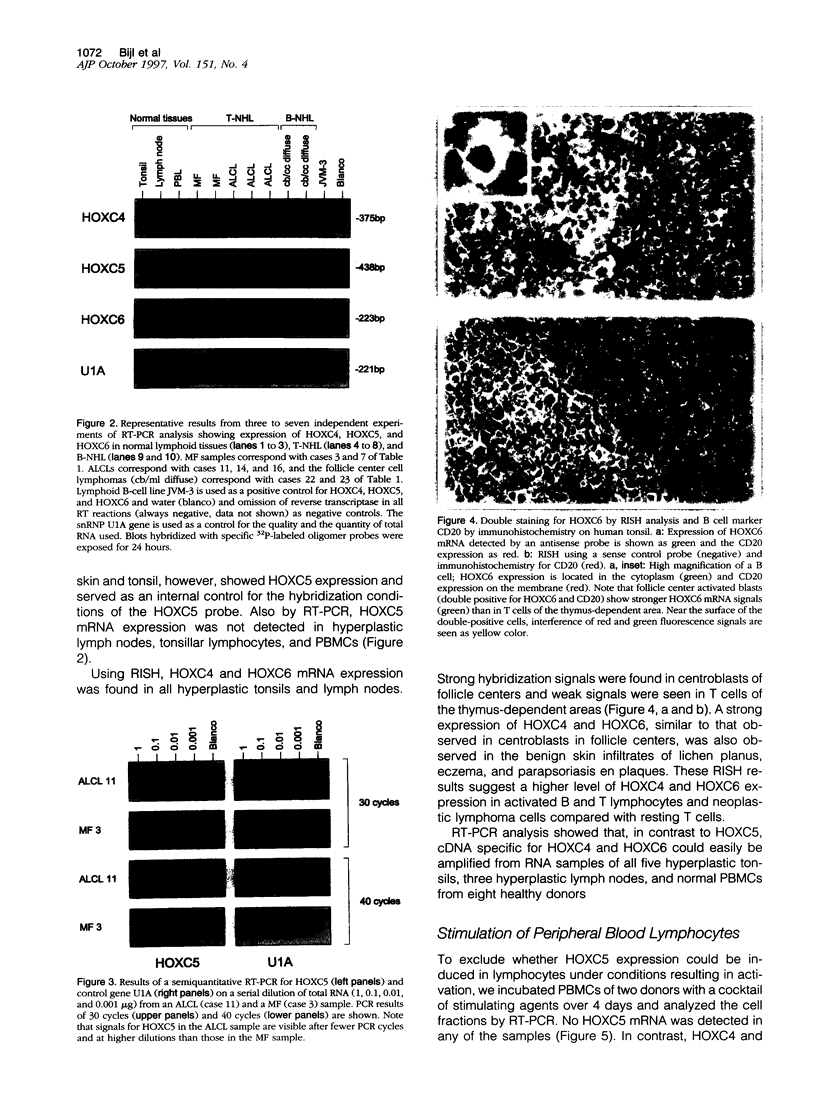
Homeobox (HOX) genes are involved in the lineage-specific differentiation of bone marrow stem cells. Recently, we reported a largely similar expression pattern of HOXC4 and HOXC6 in normal and neoplastic cells of the lymphoid lineage. In contrast, HOXC5 was specifically expressed in a subset of B-cell non-Hodgkin's lymphomas (B-NHL) but not in normal lymphocytes or lymphoid leukemias. This might suggest a role for HOXC5 in the pathogenesis of these lymphomas. In the present study the expression of HOXC4, HOXC5, and HOXC6 in primary cutaneous lymphomas was investigated. Using RNA in situ hybridization (RISH) and semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR), we found strong expression of HOXC4 and HOXC6 in all, except one, primary cutaneous lymphomas and all reactive cutaneous lymphoid infiltrates. Interestingly, a strong expression of HOXC5 in primary anaplastic CD30+ large T-cell lymphomas was found. RISH was consistently negative for HOXC5 in all other types of primary cutaneous B- and T-cell lymphomas. However, by semiquantitative RT-PCR these lymphomas showed a weak expression of HOXC5 mRNA. Therefore, we concluded that these lymphomas express low constitutive levels of HOXC5 mRNA. Furthermore, HOXC5 expression was consistently absent in reactive cutaneous lymphoid infiltrates, hyperplastic tonsils and lymph nodes, and peripheral blood lymphocytes either unstimulated or stimulated by a cocktail of CD3 and CD28 antibodies. As a strong expression of HOXC5 in primary cutaneous lymphomas was observed only in anaplastic large T-cell lymphomas and reactive control tissues lacked HOXC5 expression, these data strongly support a role for HOXC5 in the genesis of anaplastic large-T-cell lymphomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apiou F., Flagiello D., Cillo C., Malfoy B., Poupon M. F., Dutrillaux B. Fine mapping of human HOX gene clusters. Cytogenet Cell Genet. 1996;73(1-2):114–115. doi: 10.1159/000134320. [DOI] [PubMed] [Google Scholar]
- Arcioni L., Simeone A., Guazzi S., Zappavigna V., Boncinelli E., Mavilio F. The upstream region of the human homeobox gene HOX3D is a target for regulation by retinoic acid and HOX homeoproteins. EMBO J. 1992 Jan;11(1):265–277. doi: 10.1002/j.1460-2075.1992.tb05049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijl J. J., Rieger E., van Oostveen J. W., Meijer C. J., Oudejans C. B., Walboomers J. M. Quantification of biotinylated RNA probes for in situ hybridization using chemiluminescence. Histochemistry. 1994 Aug;102(1):77–82. doi: 10.1007/BF00271052. [DOI] [PubMed] [Google Scholar]
- Bijl J., van Oostveen J. W., Kreike M., Rieger E., van der Raaij-Helmer L. M., Walboomers J. M., Corte G., Boncinelli E., van den Brule A. J., Meijer C. J. Expression of HOXC4, HOXC5, and HOXC6 in human lymphoid cell lines, leukemias, and benign and malignant lymphoid tissue. Blood. 1996 Mar 1;87(5):1737–1745. [PubMed] [Google Scholar]
- Blatt C., Lotem J., Sachs L. Inhibition of specific pathways of myeloid cell differentiation by an activated Hox-2.4 homeobox gene. Cell Growth Differ. 1992 Oct;3(10):671–676. [PubMed] [Google Scholar]
- Brands J. H., Maassen J. A., van Hemert F. J., Amons R., Möller W. The primary structure of the alpha subunit of human elongation factor 1. Structural aspects of guanine-nucleotide-binding sites. Eur J Biochem. 1986 Feb 17;155(1):167–171. doi: 10.1111/j.1432-1033.1986.tb09472.x. [DOI] [PubMed] [Google Scholar]
- Celetti A., Barba P., Cillo C., Rotoli B., Boncinelli E., Magli M. C. Characteristic patterns of HOX gene expression in different types of human leukemia. Int J Cancer. 1993 Jan 21;53(2):237–244. doi: 10.1002/ijc.2910530211. [DOI] [PubMed] [Google Scholar]
- Cillo C., Barba P., Freschi G., Bucciarelli G., Magli M. C., Boncinelli E. HOX gene expression in normal and neoplastic human kidney. Int J Cancer. 1992 Jul 30;51(6):892–897. doi: 10.1002/ijc.2910510610. [DOI] [PubMed] [Google Scholar]
- De Vita G., Barba P., Odartchenko N., Givel J. C., Freschi G., Bucciarelli G., Magli M. C., Boncinelli E., Cillo C. Expression of homeobox-containing genes in primary and metastatic colorectal cancer. Eur J Cancer. 1993;29A(6):887–893. doi: 10.1016/s0959-8049(05)80432-0. [DOI] [PubMed] [Google Scholar]
- Deguchi Y., Kirschenbaum A., Kehrl J. H. A diverged homeobox gene is involved in the proliferation and lineage commitment of human hematopoietic progenitors and highly expressed in acute myelogenous leukemia. Blood. 1992 Jun 1;79(11):2841–2848. [PubMed] [Google Scholar]
- Gehring W. J., Qian Y. Q., Billeter M., Furukubo-Tokunaga K., Schier A. F., Resendez-Perez D., Affolter M., Otting G., Wüthrich K. Homeodomain-DNA recognition. Cell. 1994 Jul 29;78(2):211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Goomer R. S., Holst B. D., Wood I. C., Jones F. S., Edelman G. M. Regulation in vitro of an L-CAM enhancer by homeobox genes HoxD9 and HNF-1. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7985–7989. doi: 10.1073/pnas.91.17.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Levine M. S., Manley J. L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989 Feb 24;56(4):573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Hunt P., Krumlauf R. Hox codes and positional specification in vertebrate embryonic axes. Annu Rev Cell Biol. 1992;8:227–256. doi: 10.1146/annurev.cb.08.110192.001303. [DOI] [PubMed] [Google Scholar]
- Inamori K., Takeshita K., Chiba S., Yazaki Y., Hirai H. Identification of homeobox genes expressed in human T-lymphocytes. Biochem Biophys Res Commun. 1993 Oct 15;196(1):203–208. doi: 10.1006/bbrc.1993.2235. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Holst B. D., Minowa O., De Robertis E. M., Edelman G. M. Binding and transcriptional activation of the promoter for the neural cell adhesion molecule by HoxC6 (Hox-3.3). Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6557–6561. doi: 10.1073/pnas.90.14.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaudewitz P., Stein H., Dallenbach F., Eckert F., Bieber K., Burg G., Braun-Falco O. Primary and secondary cutaneous Ki-1+ (CD30+) anaplastic large cell lymphomas. Morphologic, immunohistologic, and clinical-characteristics. Am J Pathol. 1989 Aug;135(2):359–367. [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H. Homeobox genes in hematopoiesis. Crit Rev Oncol Hematol. 1994 Apr;16(2):145–156. doi: 10.1016/1040-8428(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Lawrence H. J., Largman C. Homeobox genes in normal hematopoiesis and leukemia. Blood. 1992 Nov 15;80(10):2445–2453. [PubMed] [Google Scholar]
- Lawrence H. J., Sauvageau G., Humphries R. K., Largman C. The role of HOX homeobox genes in normal and leukemic hematopoiesis. Stem Cells. 1996 May;14(3):281–291. doi: 10.1002/stem.140281. [DOI] [PubMed] [Google Scholar]
- Lawrence H. J., Stage K. M., Mathews C. H., Detmer K., Scibienski R., MacKenzie M., Migliaccio E., Boncinelli E., Largman C. Expression of HOX C homeobox genes in lymphoid cells. Cell Growth Differ. 1993 Aug;4(8):665–669. [PubMed] [Google Scholar]
- Magli M. C., Barba P., Celetti A., De Vita G., Cillo C., Boncinelli E. Coordinate regulation of HOX genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6348–6352. doi: 10.1073/pnas.88.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I., Stankovics J., Gallyas F. A highly sensitive one-step method for silver intensification of the nickel-diaminobenzidine endproduct of peroxidase reaction. J Histochem Cytochem. 1989 Oct;37(10):1563–1565. doi: 10.1177/37.10.2674275. [DOI] [PubMed] [Google Scholar]
- Mullink H., Vos W., Jiwa M., Horstman A., van der Valk P., Walboomers J. M., Meijer C. J. Application and comparison of silver intensification methods for the diaminobenzidine and diaminobenzidine-nickel endproduct of the peroxidation reaction in immunohistochemistry and in situ hybridization. J Histochem Cytochem. 1992 Apr;40(4):495–504. doi: 10.1177/40.4.1532404. [DOI] [PubMed] [Google Scholar]
- Nijhuis E. W., vd Wiel-van Kemenade E., Figdor C. G., van Lier R. A. Activation and expansion of tumour-infiltrating lymphocytes by anti-CD3 and anti-CD28 monoclonal antibodies. Cancer Immunol Immunother. 1990;32(4):245–250. doi: 10.1007/BF01741708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin I., Schnabel C., Catron K. M., Abate C. Hox proteins have different affinities for a consensus DNA site that correlate with the positions of their genes on the hox cluster. Mol Cell Biol. 1994 Jul;14(7):4532–4545. doi: 10.1128/mcb.14.7.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger E., Bijl J. J., van Oostveen J. W., Soyer H. P., Oudejans C. B., Jiwa N. M., Walboomers J. M., Meijer C. J. Expression of the homeobox gene HOXC4 in keratinocytes of normal skin and epithelial skin tumors is correlated with differentiation. J Invest Dermatol. 1994 Sep;103(3):341–346. doi: 10.1111/1523-1747.ep12394888. [DOI] [PubMed] [Google Scholar]
- Sauvageau G., Lansdorp P. M., Eaves C. J., Hogge D. E., Dragowska W. H., Reid D. S., Largman C., Lawrence H. J., Humphries R. K. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12223–12227. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier A. F., Gehring W. J. Functional specificity of the homeodomain protein fushi tarazu: the role of DNA-binding specificity in vivo. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1450–1454. doi: 10.1073/pnas.90.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. F., Detmer K., Mathews C. H., Hack F. M., Morgan D. A., Largman C., Lawrence H. J. Modulation of homeobox gene expression alters the phenotype of human hematopoietic cell lines. EMBO J. 1992 Mar;11(3):983–989. doi: 10.1002/j.1460-2075.1992.tb05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Pannese M., Acampora D., D'Esposito M., Boncinelli E. At least three human homeoboxes on chromosome 12 belong to the same transcription unit. Nucleic Acids Res. 1988 Jun 24;16(12):5379–5390. doi: 10.1093/nar/16.12.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchi T., Lennert K., Tu L. Y., Kikuchi M., Sato E., Stansfeld A. G., Feller A. C. Histopathology and immunohistochemistry of peripheral T cell lymphomas: a proposal for their classification. J Clin Pathol. 1987 Sep;40(9):995–1015. doi: 10.1136/jcp.40.9.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y., Komatsu N., Moriuchi T. Overexpression of the HOX4A (HOXD3) homeobox gene in human erythroleukemia HEL cells results in altered adhesive properties. Blood. 1995 May 15;85(10):2786–2794. [PubMed] [Google Scholar]
- Willemze R., Kerl H., Sterry W., Berti E., Cerroni L., Chimenti S., Diaz-Peréz J. L., Geerts M. L., Goos M., Knobler R. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997 Jul 1;90(1):354–371. [PubMed] [Google Scholar]