Abstract
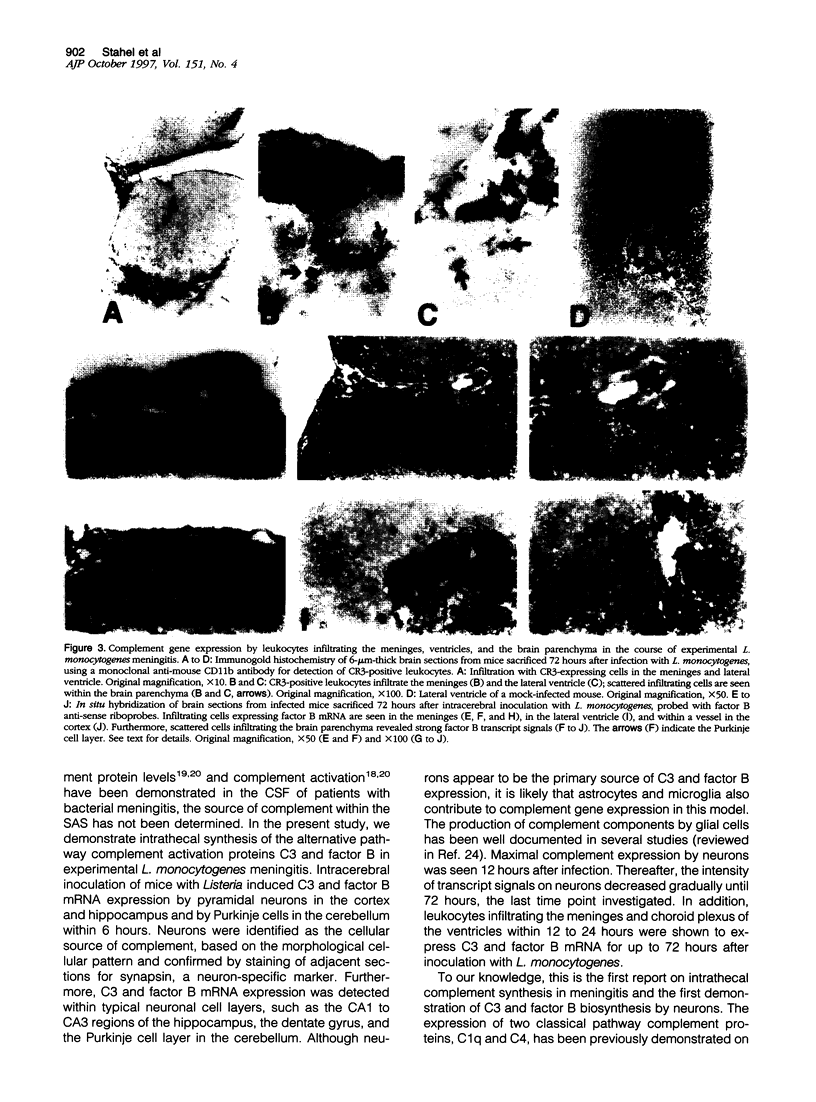
Complement has been shown to contribute to intrathecal inflammation in bacterial meningitis. However, the cellular source of complement in the infected central nervous system has not been determined. In this study, we analyzed protein and mRNA expression of two alternative pathway complement activation proteins, C3 and factor B, in the brains of mice with Listeria monocytogenes meningitis. Complement protein levels were found elevated in the cerebrospinal fluid of infected mice, compared with mock-infected animals. In the course of the disease, enhanced C3 and factor B mRNA expression was detected on pyramidal neurons and Purkinje cells within 6 hours, peaking at 12 hours and then gradually decreasing by 72 hours after infection. In addition, leukocytes infiltrating the subarachnoid space, within 12 to 24 hours, expressed mRNA for C3 and factor B. The cellular infiltration increased dramatically up to 72 hours. Intraperitoneal injection of tumor necrosis factor (TNF)-alpha up-regulated C3 and factor B mRNA expression on neurons in normal mice, suggesting that TNF-alpha may represent one cytokine regulating complement expression in this model of bacterial meningitis. However, additional mediators may be involved in regulation of intrathecal complement expression, as infected mice deficient of TNF/lymphotoxin-alpha genes did not demonstrate attenuated complement expression in the brain.
Full text
PDF



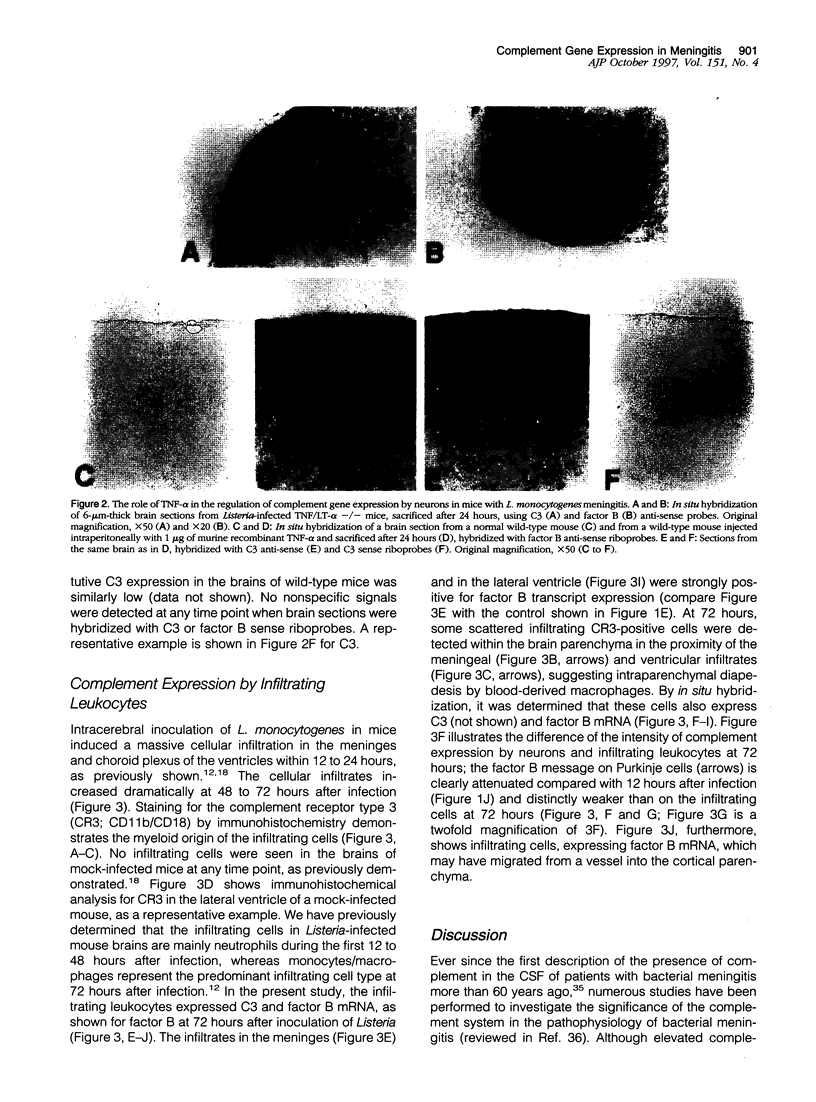


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault B. H., Colten H. R. Cellular specificity of murine renal C3 expression in two models of inflammation. Immunology. 1994 Apr;81(4):655–660. [PMC free article] [PubMed] [Google Scholar]
- Barnum S. R. Complement biosynthesis in the central nervous system. Crit Rev Oral Biol Med. 1995;6(2):132–146. doi: 10.1177/10454411950060020301. [DOI] [PubMed] [Google Scholar]
- Barnum S. R., Jones J. L., Müller-Ladner U., Samimi A., Campbell I. L. Chronic complement C3 gene expression in the CNS of transgenic mice with astrocyte-targeted interleukin-6 expression. Glia. 1996 Oct;18(2):107–117. doi: 10.1002/(SICI)1098-1136(199610)18:2<107::AID-GLIA3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bitter-Suermann D., Burger R., Brade V., Hadding U. Mouse factor B of the alternative pathway of complement activation. I. Purification, characterization, and functional behavior. J Immunol. 1976 Nov;117(5 PT2):1799–1804. [PubMed] [Google Scholar]
- Boje K. M. Cerebrovascular permeability changes during experimental meningitis in the rat. J Pharmacol Exp Ther. 1995 Sep;274(3):1199–1203. [PubMed] [Google Scholar]
- Dujardin B. C., Driedijk P. C., Roijers A. F., Out T. A. The determination of the complement components C1q, C4 and C3 in serum and cerebrospinal fluid by radioimmunoassay. J Immunol Methods. 1985 Jun 25;80(2):227–237. doi: 10.1016/0022-1759(85)90024-9. [DOI] [PubMed] [Google Scholar]
- Durand M. L., Calderwood S. B., Weber D. J., Miller S. I., Southwick F. S., Caviness V. S., Jr, Swartz M. N. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993 Jan 7;328(1):21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- Eugster H. P., Muller M., Karrer U., Car B. D., Schnyder B., Eng V. M., Woerly G., Le Hir M., di Padova F., Aguet M. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double-deficient mice. Int Immunol. 1996 Jan;8(1):23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- Faustmann P. M., Krause D., Dux R., Dermietzel R. Morphological study in the early stages of complement C5a fragment-induced experimental meningitis: activation of macrophages and astrocytes. Acta Neuropathol. 1995;89(3):239–247. doi: 10.1007/BF00309339. [DOI] [PubMed] [Google Scholar]
- Frei K., Nadal D., Pfister H. W., Fontana A. Listeria meningitis: identification of a cerebrospinal fluid inhibitor of macrophage listericidal function as interleukin 10. J Exp Med. 1993 Oct 1;178(4):1255–1261. doi: 10.1084/jem.178.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E. G., Banks W. A., Kastin A. J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993 Sep;47(2):169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Ishikawa N., Nonaka M., Wetsel R. A., Colten H. R. Murine complement C2 and factor B genomic and cDNA cloning reveals different mechanisms for multiple transcripts of C2 and B. J Biol Chem. 1990 Nov 5;265(31):19040–19046. [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Hengstler B., Bray M. A., Zak O. Inhibition of complement-factor-5a-induced inflammatory reactions by prostaglandin E2 in experimental meningitis. J Infect Dis. 1989 Oct;160(4):715–719. doi: 10.1093/infdis/160.4.715. [DOI] [PubMed] [Google Scholar]
- Kossmann T., Stahel P. F., Morganti-Kossmann M. C., Jones J. L., Barnum S. R. Elevated levels of the complement components C3 and factor B in ventricular cerebrospinal fluid of patients with traumatic brain injury. J Neuroimmunol. 1997 Mar;73(1-2):63–69. doi: 10.1016/s0165-5728(96)00164-6. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Frei K., Kam-Hansen S., Zinkernagel R. M., Fontana A. Tumor necrosis factor alpha in cerebrospinal fluid during bacterial, but not viral, meningitis. Evaluation in murine model infections and in patients. J Exp Med. 1988 May 1;167(5):1743–1748. doi: 10.1084/jem.167.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal D., Leppert D., Frei K., Gallo P., Lamche H., Fontana A. Tumour necrosis factor-alpha in infectious meningitis. Arch Dis Child. 1989 Sep;64(9):1274–1279. doi: 10.1136/adc.64.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. W., Fontana A., Täuber M. G., Tomasz A., Scheld W. M. Mechanisms of brain injury in bacterial meningitis: workshop summary. Clin Infect Dis. 1994 Sep;19(3):463–479. doi: 10.1093/clinids/19.3.463. [DOI] [PubMed] [Google Scholar]
- Quagliarello V. J., Scheld W. M. Treatment of bacterial meningitis. N Engl J Med. 1997 Mar 6;336(10):708–716. doi: 10.1056/NEJM199703063361007. [DOI] [PubMed] [Google Scholar]
- Quagliarello V., Scheld W. M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992 Sep 17;327(12):864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- Roth J., Saremaslani P., Warhol M. J., Heitz P. U. Improved accuracy in diagnostic immunohistochemistry, lectin histochemistry and in situ hybridization using a gold-labeled horseradish peroxidase antibody and silver intensification. Lab Invest. 1992 Aug;67(2):263–269. [PubMed] [Google Scholar]
- Rozovsky I., Morgan T. E., Willoughby D. A., Dugichi-Djordjevich M. M., Pasinetti G. M., Johnson S. A., Finch C. E. Selective expression of clusterin (SGP-2) and complement C1qB and C4 during responses to neurotoxins in vivo and in vitro. Neuroscience. 1994 Oct;62(3):741–758. doi: 10.1016/0306-4522(94)90473-1. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Sande S., Cioffe C., Wolpe S., Sherry B., Cerami A., Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990 Feb 1;171(2):439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebach J., Bartholdi D., Frei K., Spanaus K. S., Ferrero E., Widmer U., Isenmann S., Strieter R. M., Schwab M., Pfister H. Experimental Listeria meningoencephalitis. Macrophage inflammatory protein-1 alpha and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995 Nov 1;155(9):4367–4375. [PubMed] [Google Scholar]
- Spanaus K. S., Nadal D., Pfister H. W., Seebach J., Widmer U., Frei K., Gloor S., Fontana A. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J Immunol. 1997 Feb 15;158(4):1956–1964. [PubMed] [Google Scholar]
- Stahel P. F., Frei K., Eugster H. P., Fontana A., Hummel K. M., Wetsel R. A., Ames R. S., Barnum S. R. TNF-alpha-mediated expression of the receptor for anaphylatoxin C5a on neurons in experimental Listeria meningoencephalitis. J Immunol. 1997 Jul 15;159(2):861–869. [PubMed] [Google Scholar]
- Stahel P. F., Nadal D., Pfister H. W., Paradisis P. M., Barnum S. R. Complement C3 and factor B cerebrospinal fluid concentrations in bacterial and aseptic meningitis. Lancet. 1997 Jun 28;349(9069):1886–1887. doi: 10.1016/S0140-6736(05)63877-9. [DOI] [PubMed] [Google Scholar]
- Sáez-Llorens X., Jafari H. S., Severien C., Parras F., Olsen K. D., Hansen E. J., Singer I. I., McCracken G. H., Jr Enhanced attenuation of meningeal inflammation and brain edema by concomitant administration of anti-CD18 monoclonal antibodies and dexamethasone in experimental Haemophilus meningitis. J Clin Invest. 1991 Dec;88(6):2003–2011. doi: 10.1172/JCI115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., Frenette P. S., Hynes R. O., Wagner D. D., Mayadas T. N. Cytokine-induced meningitis is dramatically attenuated in mice deficient in endothelial selectins. J Clin Invest. 1996 Jun 1;97(11):2485–2490. doi: 10.1172/JCI118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend G. C., Scheld W. M. Adjunctive therapy for bacterial meningitis: rationale for use, current status, and prospects for the future. Clin Infect Dis. 1993 Nov;17 (Suppl 2):S537–S549. doi: 10.1093/clinids/17.supplement_2.s537. [DOI] [PubMed] [Google Scholar]
- Townsend G. C., Scheld W. M. The use of corticosteroids in the management of bacterial meningitis in adults. J Antimicrob Chemother. 1996 Jun;37(6):1051–1061. doi: 10.1093/jac/37.6.1051. [DOI] [PubMed] [Google Scholar]
- Tuomanen E. I., Saukkonen K., Sande S., Cioffe C., Wright S. D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989 Sep 1;170(3):959–969. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Hengstler B., Zak O., Tomasz A. The role of complement in inflammation during experimental pneumococcal meningitis. Microb Pathog. 1986 Feb;1(1):15–32. doi: 10.1016/0882-4010(86)90028-8. [DOI] [PubMed] [Google Scholar]
- Täuber M. G., Borschberg U., Sande M. A. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J Infect Dis. 1988 Mar;157(3):456–464. doi: 10.1093/infdis/157.3.456. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989 Dec 1;170(6):1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel R. A., Lundwall A., Davidson F., Gibson T., Tack B. F., Fey G. H. Structure of murine complement component C3. II. Nucleotide sequence of cloned complementary DNA coding for the alpha chain. J Biol Chem. 1984 Nov 25;259(22):13857–13862. [PubMed] [Google Scholar]
- Zwahlen A., Nydegger U. E., Vaudaux P., Lambert P. H., Waldvogel F. A. Complement-mediated opsonic activity in normal and infected human cerebrospinal fluid: early response during bacterial meningitis. J Infect Dis. 1982 May;145(5):635–646. doi: 10.1093/infdis/145.2.635. [DOI] [PubMed] [Google Scholar]