Abstract
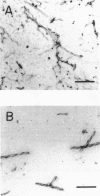
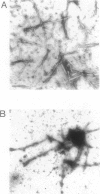
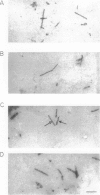
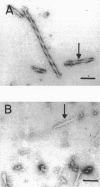
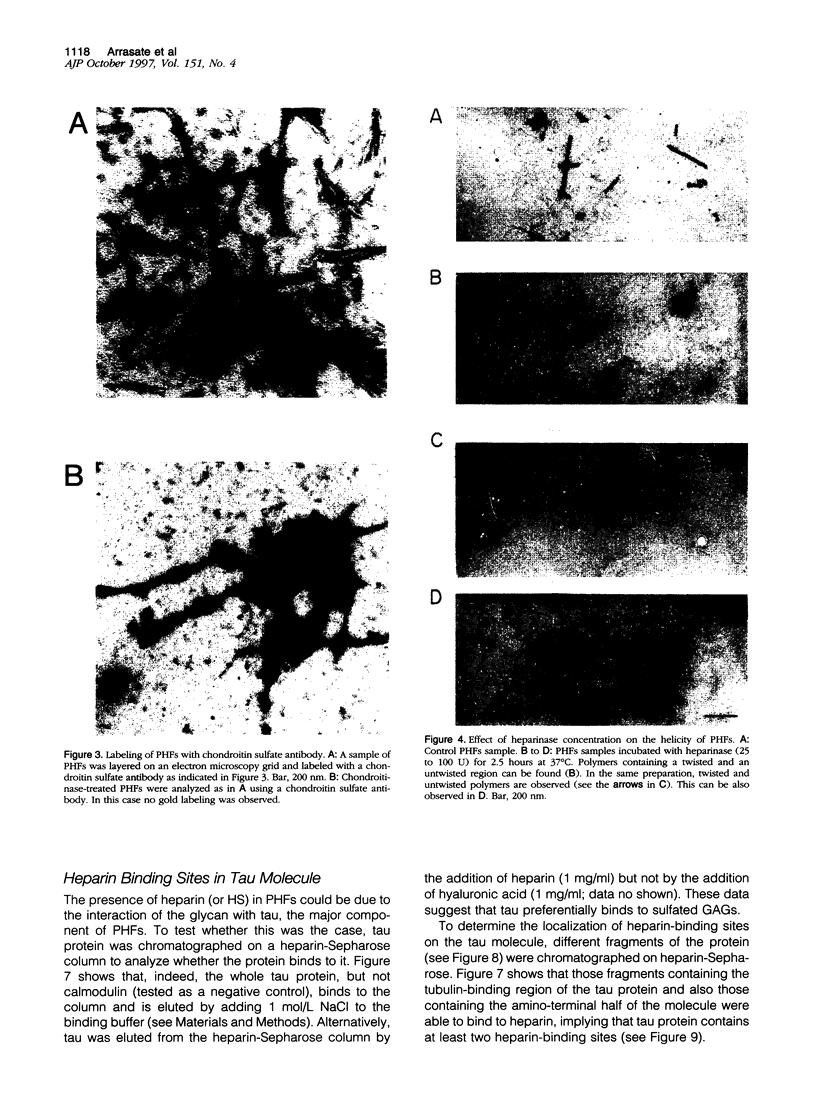
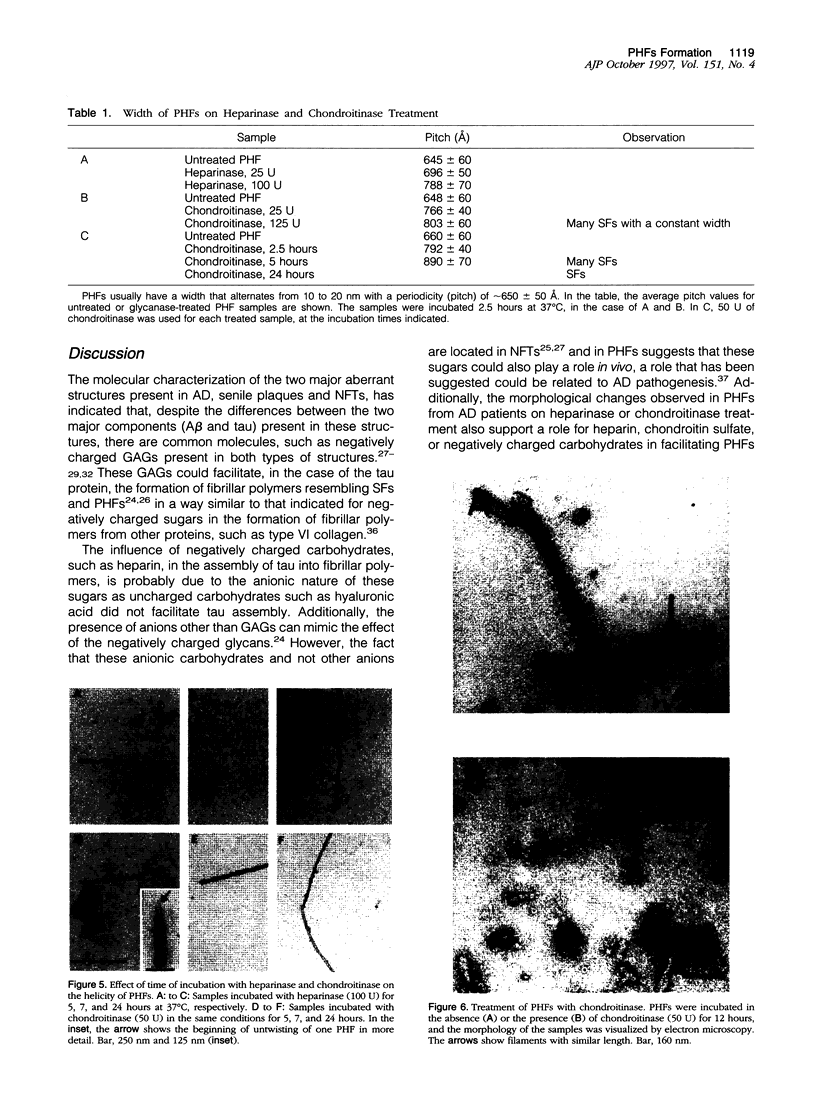
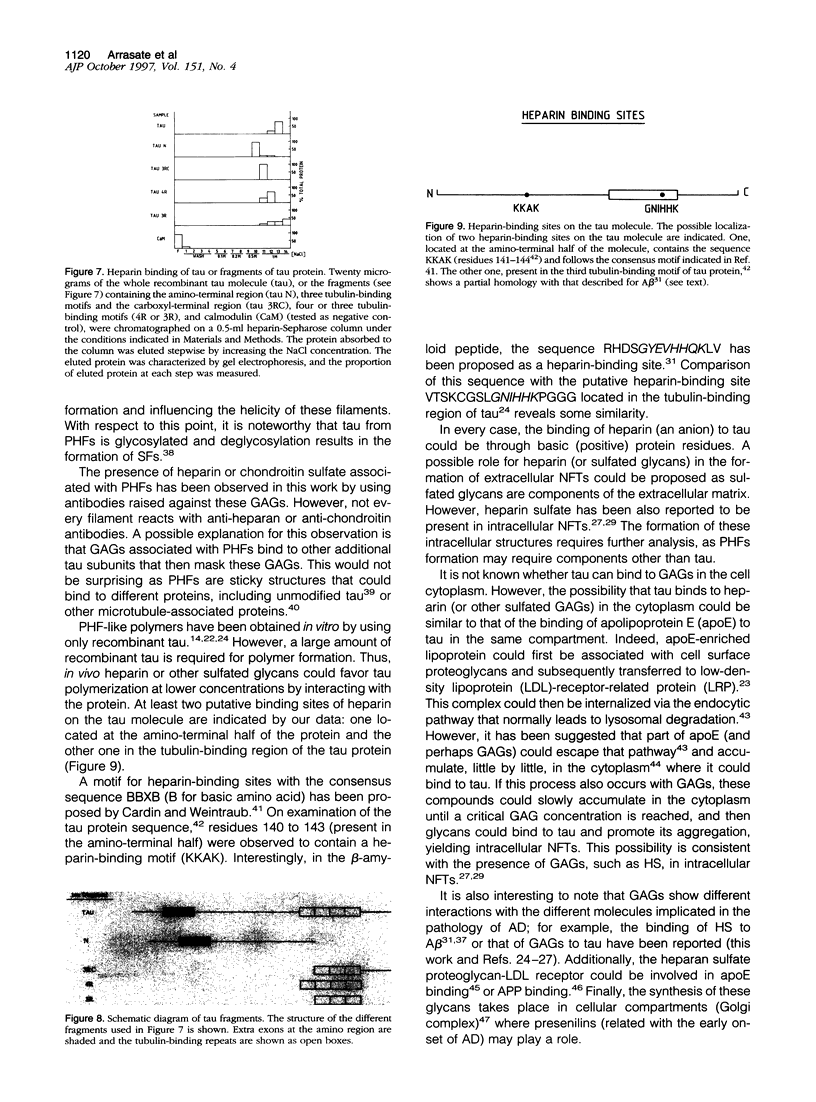
It is known from previous work that tau is the main component of paired helical filaments (PHFs) and that it can assemble in vitro into polymers resembling PHFs when high concentrations of protein are used. In the search for molecules that can facilitate tau polymerization, a component of neurofibrillary tangles, heparan sulfate (or its more sulfated form, heparin), and other glycosaminoglycans have been tested. Glycosaminoglycans, in the sulfated but not in the unsulfated form, facilitate not only tau assembly but also the formation of polymers resembling PHFs. Conversely, PHFs were found to contain heparan sulfate and chondroitin sulfate. Heparinase or chondroitinase treatment of PHFs results in the formation of straight structures. All of these results suggest a role for sulfated glycosaminoglycans in determining the helicity of PHFs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A. C., Grundke-Iqbal I., Iqbal K. Alzheimer's disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996 Jul;2(7):783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- Alonso A. D., Grundke-Iqbal I., Barra H. S., Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U S A. 1997 Jan 7;94(1):298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., Hyman B. T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992 Mar;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Bellosta S., Nathan B. P., Orth M., Dong L. M., Mahley R. W., Pitas R. E. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995 Nov 10;270(45):27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Olesen O. F., Jakes R., Goedert M. The microtubule binding repeats of tau protein assemble into filaments like those found in Alzheimer's disease. FEBS Lett. 1992 Sep 7;309(2):199–202. doi: 10.1016/0014-5793(92)81094-3. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Olesen O. F., Smith M. J., Jakes R., Goedert M. Assembly of Alzheimer-like filaments from full-length tau protein. FEBS Lett. 1994 Jan 10;337(2):135–138. doi: 10.1016/0014-5793(94)80260-2. [DOI] [PubMed] [Google Scholar]
- DeWitt D. A., Silver J., Canning D. R., Perry G. Chondroitin sulfate proteoglycans are associated with the lesions of Alzheimer's disease. Exp Neurol. 1993 Jun;121(2):149–152. doi: 10.1006/exnr.1993.1081. [DOI] [PubMed] [Google Scholar]
- Dudek S. M., Johnson G. V. Transglutaminase catalyzes the formation of sodium dodecyl sulfate-insoluble, Alz-50-reactive polymers of tau. J Neurochem. 1993 Sep;61(3):1159–1162. doi: 10.1111/j.1471-4159.1993.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Spillantini M. G., Hasegawa M., Smith M. J., Crowther R. A. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996 Oct 10;383(6600):550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D. M., Pitas R. E., Kilbridge J., Nathan B., Mahley R. W., Bu G., Schwartz A. L. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y., Nukina N., Miura R., Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem. 1986 Jun;99(6):1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas M. Z., Moir R. D., Rebeck G. W., Bush A. I., Argraves W. S., Tanzi R. E., Hyman B. T., Strickland D. K. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995 Jul 28;82(2):331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Kovacs D. M., Fausett H. J., Page K. J., Kim T. W., Moir R. D., Merriam D. E., Hollister R. D., Hallmark O. G., Mancini R., Felsenstein K. M. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996 Feb;2(2):224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- Krieger M., Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Structural stability of paired helical filaments requires microtubule-binding domains of tau: a model for self-association. Neuron. 1991 May;6(5):717–728. doi: 10.1016/0896-6273(91)90169-z. [DOI] [PubMed] [Google Scholar]
- Kuo H. J., Keene D. R., Glanville R. W. The macromolecular structure of type-VI collagen. Formation and stability of filaments. Eur J Biochem. 1995 Sep 1;232(2):364–372. [PubMed] [Google Scholar]
- Lovestone S., Anderton B. H., Hartley C., Jensen T. G., Jorgensen A. L. The intracellular fate of apolipoprotein E is tau dependent and apoe allele-specific. Neuroreport. 1996 Apr 10;7(5):1005–1008. doi: 10.1097/00001756-199604100-00010. [DOI] [PubMed] [Google Scholar]
- Medina M., Montejo de Garcini E., Avila J. The role of tau phosphorylation in transfected COS-1 cells. Mol Cell Biochem. 1995 Jul 5;148(1):79–88. doi: 10.1007/BF00929506. [DOI] [PubMed] [Google Scholar]
- Montejo de Garcini E., Avila J. In vitro conditions for the self-polymerization of the microtubule-associated protein, tau factor. J Biochem. 1987 Dec;102(6):1415–1421. doi: 10.1093/oxfordjournals.jbchem.a122187. [DOI] [PubMed] [Google Scholar]
- Montejo de Garcini E., Carrascosa J. L., Correas I., Nieto A., Avila J. Tau factor polymers are similar to paired helical filaments of Alzheimer's disease. FEBS Lett. 1988 Aug 15;236(1):150–154. doi: 10.1016/0014-5793(88)80304-1. [DOI] [PubMed] [Google Scholar]
- Montejo de Garcini E., Serrano L., Avila J. Self assembly of microtubule associated protein tau into filaments resembling those found in Alzheimer disease. Biochem Biophys Res Commun. 1986 Dec 15;141(2):790–796. doi: 10.1016/s0006-291x(86)80242-x. [DOI] [PubMed] [Google Scholar]
- Nieto A., Correas I., Montejo de Garcini E., Avila J. A modified form of microtubule-associated tau protein is the main component of paired helical filaments. Biochem Biophys Res Commun. 1988 Jul 29;154(2):660–667. doi: 10.1016/0006-291x(88)90190-8. [DOI] [PubMed] [Google Scholar]
- Perry G., Siedlak S. L., Richey P., Kawai M., Cras P., Kalaria R. N., Galloway P. G., Scardina J. M., Cordell B., Greenberg B. D. Association of heparan sulfate proteoglycan with the neurofibrillary tangles of Alzheimer's disease. J Neurosci. 1991 Nov;11(11):3679–3683. doi: 10.1523/JNEUROSCI.11-11-03679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollanen M. S., Markiewicz P., Bergeron C., Goh M. C. Twisted ribbon structure of paired helical filaments revealed by atomic force microscopy. Am J Pathol. 1994 May;144(5):869–873. [PMC free article] [PubMed] [Google Scholar]
- Praprotnik D., Smith M. A., Richey P. L., Vinters H. V., Perry G. Filament heterogeneity within the dystrophic neurites of senile plaques suggests blockage of fast axonal transport in Alzheimer's disease. Acta Neuropathol. 1996;91(3):226–235. doi: 10.1007/s004010050420. [DOI] [PubMed] [Google Scholar]
- Pérez M., Valpuesta J. M., Medina M., Montejo de Garcini E., Avila J. Polymerization of tau into filaments in the presence of heparin: the minimal sequence required for tau-tau interaction. J Neurochem. 1996 Sep;67(3):1183–1190. doi: 10.1046/j.1471-4159.1996.67031183.x. [DOI] [PubMed] [Google Scholar]
- Small D. H., Nurcombe V., Reed G., Clarris H., Moir R., Beyreuther K., Masters C. L. A heparin-binding domain in the amyloid protein precursor of Alzheimer's disease is involved in the regulation of neurite outgrowth. J Neurosci. 1994 Apr;14(4):2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. H., Williamson T., Reed G., Clarris H., Beyreuther K., Masters C. L., Nurcombe V. The role of heparan sulfate proteoglycans in the pathogenesis of Alzheimer's disease. Ann N Y Acad Sci. 1996 Jan 17;777:316–321. doi: 10.1111/j.1749-6632.1996.tb34439.x. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Mar H., Nochlin D., Kimata K., Kato M., Suzuki S., Hassell J., Wight T. N. The presence of heparan sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol. 1988 Dec;133(3):456–463. [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Sekiguchi R., Nochlin D., Fraser P., Kimata K., Mizutani A., Arai M., Schreier W. A., Morgan D. G. An important role of heparan sulfate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar A beta-amyloid in rat brain. Neuron. 1994 Jan;12(1):219–234. doi: 10.1016/0896-6273(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Su J. H., Cummings B. J., Cotman C. W. Localization of heparan sulfate glycosaminoglycan and proteoglycan core protein in aged brain and Alzheimer's disease. Neuroscience. 1992 Dec;51(4):801–813. doi: 10.1016/0306-4522(92)90521-3. [DOI] [PubMed] [Google Scholar]
- Troncoso J. C., Costello A., Watson A. L., Jr, Johnson G. V. In vitro polymerization of oxidized tau into filaments. Brain Res. 1993 Jun 11;613(2):313–316. doi: 10.1016/0006-8993(93)90918-d. [DOI] [PubMed] [Google Scholar]
- Wang J. Z., Grundke-Iqbal I., Iqbal K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer's disease. Nat Med. 1996 Aug;2(8):871–875. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- Wille H., Drewes G., Biernat J., Mandelkow E. M., Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992 Aug;118(3):573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Binder L. I. Polymerization of microtubule-associated protein tau under near-physiological conditions. J Biol Chem. 1995 Oct 13;270(41):24306–24314. doi: 10.1074/jbc.270.41.24306. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]